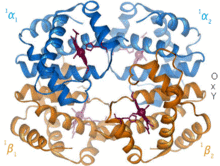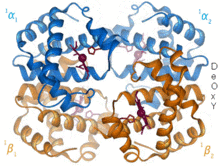
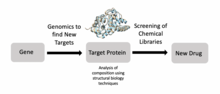
Structural biology
At lower resolutions, tools such as FIB-SEM tomography have allowed for greater understanding of cells and their organelles in 3-dimensions, and how each hierarchical level of various extracellular matrices contributes to function (for example in bone).
In the past few years it has also become possible to predict highly accurate physical molecular models to complement the experimental study of biological structures.
Francis Crick and James Watson modeled the double helical structure of DNA using this same technique in 1953 and received the Nobel Prize in Medicine along with Wilkins in 1962.
[14] In the late 1930s and early 1940s, the combination of work done by Isidor Rabi, Felix Bloch, and Edward Mills Purcell led to the development of nuclear magnetic resonance (NMR).
[16] Since then, cryo-EM has emerged as an increasingly popular technique to determine three-dimensional, high resolution structures of biological images.
[18] Recently, protein structure prediction[broken anchor] was significantly improved by a new machine learning method called AlphaFold.
Structural biologists have made significant contributions towards understanding the molecular components and mechanisms underlying human diseases.
[21] In addition to amyloid proteins, scientists have used cryo-EM to produce high resolution models of tau filaments in the brain of Alzheimer's patients which may help develop better treatments in the future.
For example, structural biology tools have enabled virologists to understand how the HIV envelope allows the virus to evade human immune responses.
For example, researchers have used structural biology to better understand Met, a protein encoded by a protooncogene that is an important drug target in cancer.