Sulfite process
[3] The first sulphite mill in the United States was the Richmond Paper Company in Rumford, Rhode Island in the mid-1880s.
The invention of the recovery boiler by G. H. Tomlinson in the early 1930s [2] allowed kraft mills to recycle almost all of their pulping chemicals.
The pulping liquor for most sulfite mills is generated by treating various bases (alkali metal or alkaline earth hydroxides) with sulfur dioxide: Similar reactions are effected with divalent cations (Mg2+, Ca2+) and using carbonates in place of hydroxide.
[8] The concentrated brown liquor is burned in a recovery boiler, producing magnesium oxide and sulfur dioxide, both of which are recovered from the flue gases.
Pulp washers, using countercurrent flow, remove the spent cooking chemicals and degraded lignin and hemicellulose.
Recent developments in Chemrec's black liquor gasification process, adapting the technology to use in the sulfite pulping process, could make second generation biofuels production an alternative to the conventional recovery boiler technology.
[9] Around 1906 Gösta Ekström a Swedish engineer patented a process of ethanol generation from the residual 2-2.5% fermentable hexose sugars in the spent liquor.
Sulfite pulp remains an important commodity, especially for specialty papers and as a source of cellulose for non-paper applications.
Methylcellulose and other cellulose ether derivatives are used in a wide range of everyday products from adhesives to baked goods to pharmaceuticals.
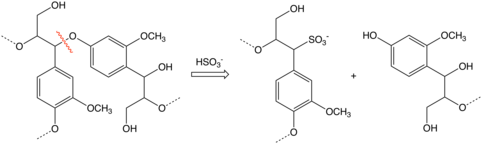
Chief among sulfite process byproducts are lignosulfonates, which find a wide variety of uses where a relatively inexpensive agent is needed to make a water dispersion of a water-insoluble material.
[14] Oxidation of lignosulfonates was used to produce vanillin (artificial vanilla), and this process is still used by one supplier (Borregaard, Norway) while all North American production by this route ceased in the 1990s.