Sulfur vulcanization
[1] Sulfur forms cross-linking bridges between sections of polymer chains which affects the mechanical and electronic properties.
[citation needed] Many products are made with vulcanized rubber, including tires, shoe soles, hoses, and conveyor belts.
Even with natural rubber, large amounts of sulfur as well as high temperatures and prolonged heating periods are necessary, with the end products often being of an unsatisfactory quality.
Since the early 1900s, various chemical additives have been developed to improve the speed and efficiency of vulcanization, as well as to control the nature of the cross-linking.
[7][8] Note that these are merely the additives used for vulcanization and that other compounds may also be added to the rubber, such as fillers, tackifiers, polymer stabilizers and antiozonants.
It is also possible to replace sulfur with other sulfur-donating compounds, for example accelerants bearing disulfide groups, in what is often termed "efficient vulcanization" (EV).
[15] It remains a moderately fast curing agent giving sulfur chains of a medium length, but its relatively short induction period can be a disadvantage.
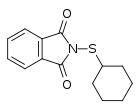
Oxidative dimerization of MBT gives mercaptobenzothiazole disulfide (MBTS), and sulfenamide derivatives are produced by reacting this with primary amines like cyclohexylamine or tert-butylamine.
They act to boost the cure speed and increase cross-link density, but also shorten the induction time, which can lead to premature vulcanization.
[7] Chemically, they consist mainly of thio-carbonyl species such as thiurams, dithiocarbamates, xanthates and organic thioureas; aromatic guanidines are also used.
Activators consist of various metal salts, fatty acids, as well as nitrogen-containing bases, the most important these being zinc oxide.
To ensure high-quality vulcanization, the rubber, sulfur, accelerants, activators and other compounds are blended to give a homogeneous mixture.
At these temperatures vulcanization can begin prematurely, which is often undesirable, as the mixture may still need to be pumped and moulded into its final form before it sets solid.
The juice of a local vine, Ipomoea alba, was then mixed with this latex to create processed rubber as early as 1600 BCE.
[25] In the Western world, rubber remained a curiosity, although it was eventually used to produce waterproofed products, such as Mackintosh rainwear, beginning in the early 1800s.
He describes the scene in a rubber factory where his brother worked: The inventor made experiments to ascertain the effect of heat on the same compound that had decomposed in the mail-bags and other articles.
He was surprised to find that the specimen, being carelessly brought into contact with a hot stove, charred like leather.Goodyear goes on to describe how his discovery was not readily accepted.
On ascertaining to a certainty that he had found the object of his search and much more, and that the new substance was proof against cold and the solvent of the native gum, he felt himself amply repaid for the past, and quite indifferent to the trials of the future.The discovery of the rubber-sulfur reaction revolutionized the use and applications of rubber, changing the face of the industrial world.
This practice was acceptable only at moderate pressures, but above a certain point, machine designers were forced to compromise between the extra friction generated by tighter packing and greater leakage of steam.
These exceptional qualities, combined with good durability and lack of stickiness, were critical for an effective sealing material.
[30] In 1905 George Oenslager discovered that a derivative of aniline called thiocarbanilide accelerated the reaction of sulfur with rubber, leading to shorter cure times and reducing energy consumption.


N.B. In this image, the degree of crosslinking is exaggerated for illustrative purposes.