Aniline
Aniline (from Portuguese anil 'indigo shrub', and -ine indicating a derived substance)[6] is an organic compound with the formula C6H5NH2.
The amino group in aniline is flatter (i.e., it is a "shallower pyramid") than that in an aliphatic amine, owing to conjugation of the lone pair with the aryl substituent.
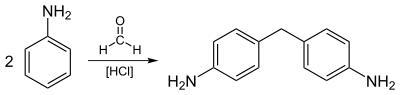
Industrial aniline production involves hydrogenation of nitrobenzene (typically at 200–300 °C) in the presence of metal catalysts:[15] Approximately 4 billion kilograms are produced annually.
[7] Alternatively, using Buchwald-Hartwig coupling or Ullmann reaction approaches, aryl halides can be aminated with aqueous or gaseous ammonia.
In alkaline solution, azobenzene results, whereas arsenic acid produces the violet-coloring matter violaniline.
Chromic acid converts it into quinone, whereas chlorates, in the presence of certain metallic salts (especially of vanadium), give aniline black.
Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
Boiled with carbon disulfide, it gives sulfocarbanilide (diphenylthiourea) (S=C(−NH−C6H5)2), which may be decomposed into phenyl isothiocyanate (C6H5−N=C=S), and triphenyl guanidine (C6H5−N=C(−NH−C6H5)2).
This diazonium salt can also be reacted with NaNO2 and phenol to produce a dye known as benzeneazophenol, in a process called coupling.
The benzene diazonium salt is formed as major product alongside the byproducts water and sodium chloride.
The diamines are condensed with phosgene to give methylene diphenyl diisocyanate, a precursor to urethane polymers.
Kerrigan's 2016 Agaricus of North America P45: (Referring to Schaffer's reaction) "In fact I recommend switching to the following modified test.
Frank (1988) developed an alternative formulation in which aniline oil is combined with glacial acetic acid (GAA, essentially distilled vinegar) in a 50:50 solution.
In 1834, Friedlieb Runge isolated a substance from coal tar that turned a beautiful blue color when treated with chloride of lime.
[28] In 1840, Carl Julius Fritzsche (1808–1871) treated indigo with caustic potash and obtained an oil that he named aniline, after an indigo-yielding plant, anil (Indigofera suffruticosa).
[32] In 1856, while trying to synthesise quinine, von Hofmann's student William Henry Perkin discovered mauveine.
[35] In the late 19th century, derivatives of aniline such as acetanilide and phenacetin emerged as analgesic drugs, with their cardiac-suppressive side effects often countered with caffeine.
[36] During the first decade of the 20th century, while trying to modify synthetic dyes to treat African sleeping sickness, Paul Ehrlich – who had coined the term chemotherapy for his magic bullet approach to medicine – failed and switched to modifying Béchamp's atoxyl, the first organic arsenical drug, and serendipitously obtained a treatment for syphilis – salvarsan – the first successful chemotherapy agent.
Salvarsan's targeted microorganism, not yet recognized as a bacterium, was still thought to be a parasite, and medical bacteriologists, believing that bacteria were not susceptible to the chemotherapeutic approach, overlooked Alexander Fleming's report in 1928 on the effects of penicillin.
Gerhard Domagk identified as an antibacterial a red azo dye, introduced in 1935 as the first antibacterial drug, prontosil, soon found at Pasteur Institute to be a prodrug degraded in vivo into sulfanilamide – a colorless intermediate for many, highly colorfast azo dyes – already with an expired patent, synthesized in 1908 in Vienna by the researcher Paul Gelmo for his doctoral research.
[37] Medications in high demand during World War II (1939–45), these first miracle drugs, chemotherapy of wide effectiveness, propelled the American pharmaceutics industry.
[38] In 1939, at Oxford University, seeking an alternative to sulfa drugs, Howard Florey developed Fleming's penicillin into the first systemic antibiotic drug, penicillin G. (Gramicidin, developed by René Dubos at Rockefeller Institute in 1939, was the first antibiotic, yet its toxicity restricted it to topical use.)
After World War II, Cornelius P. Rhoads introduced the chemotherapeutic approach to cancer treatment.
[39] Some early American rockets, such as the Aerobee and WAC Corporal, used a mixture of aniline and furfuryl alcohol as a fuel, with nitric acid as an oxidizer.
The accumulation of oxidative DNA damages in the spleen following exposure to aniline may increase mutagenic events that underlie tumorigenesis.