Summation theorems (biochemistry)
In metabolic control analysis, a variety of theorems have been discovered and discussed in the literature.
[1][2][3][4] The most well known of these are flux and concentration control coefficient summation relationships.
These theorems are the result of the stoichiometric structure and mass conservation properties of biochemical networks.
[5][6] Equivalent theorems have not been found, for example, in electrical or economic systems.
The summation of the flux and concentration control coefficients were discovered independently by the Kacser/Burns group[7] and the Heinrich/Rapoport group[8] in the early 1970s and late 1960s.
If we define the control coefficients using enzyme concentration, then the summation theorems are written as: However these theorems depend on the assumption that reaction rates are proportional to enzyme concentration.
An alternative way to write the theorems is to use control coefficients that are defined with respect to the local rates which is therefore independent of how rates respond to changes in enzyme concentration: Although originally derived for simple linear chains of enzyme catalyzed reactions, it became apparent that the theorems applied to pathways of any structure including pathways with complex regulation involving feedback control.
[9][10] There are different ways to derive the summation theorems.
One is analytical and rigorous using a combination of linear algebra and calculus.
are fixed species so that the system can achieve a steady-state.
Let the pathway be at steady-state and imagine increasing the concentration of enzyme,
The effect of this is to increase the steady-state levels of S and flux, J.
such that the change in S is restored to the original value it had at steady-state.
The net effect of these two changes is by definition,
For the system we can compute the overall change in flux or species concentration by adding the two control coefficient terms, thus:
We can also look at what is happening locally at every reaction step for which there will be two: one for
However, because the enzyme elasticity is equal to one, these reduce to:
We can substitute these expressions into the system equations to give:
Note that at steady state the change in
The summation theorems can be interpreted in various ways.
In the past, control of a pathway was considered to be located at one point only, called the master reaction or rate limiting step.
The summation theorem suggests this does not necessarily have to be the case.
The flux summation theorem also suggests that there is a total amount of flux control in a pathway such that if one step gains control another step most lose control.
In order for a biological cell to have any appreciable control over a pathway via changes in gene expression, some concentration of flux control at a small number of sites will be necessary.
For example, in mammalian cancer cell lines, it has been shown[12] that flux control is concentrated at four sites: glucose import, hexokinase, phosphofructokinase, and lactate export.
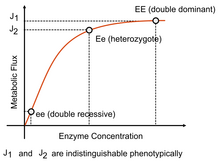
Moreover, Kacser and Burns[13] suggested that since the flux–enzyme relationship is somewhat hyperbolic, and that for most enzymes, the wild-type diploid level of enzyme activity occurs where the curve is reaching a point in the curve where changes have little effect, then since a heterozygote of the wild-type with a null mutant will have half the enzyme activity it will not exhibit a noticeably reduced flux.
Therefore, the wild type appears dominant and the mutant recessive because of the system characteristics of a metabolic pathway.
Although originally suggested by Sewall Wright,[14][15] the development of metabolic control analysis put the idea on a more sound theoretical footing.
The flux summation theorem in particular is consistent with the flux summation theorem for large systems.
This is particularly noticeable in a linear chain of enzyme reactions where, given a metabolite located in the center of the pathway, an increase in expression of any enzyme upstream of the metabolite will cause the metabolite to increase in concentration.