Superantigen
In normal circumstances it is released locally in low levels and helps the immune system defeat pathogens.
However, when it is systemically released in the blood and in high levels (due to mass T-cell activation resulting from the SAg binding), it can cause severe and life-threatening symptoms, including septic shock and multiple organ failure.
[7] Crystal structures of the enterotoxins reveals that they are compact, ellipsoidal proteins sharing a characteristic two-domain folding pattern comprising an NH2-terminal β barrel globular domain known as the oligosaccharide / oligonucleotide fold, a long α-helix that diagonally spans the center of the molecule, and a COOH-terminal globular domain.
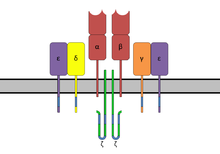
[8] Superantigens bind first to the MHC class II and then coordinate to the variable alpha- or beta chain of T-cell Receptors (TCR)[6][9][10] SAgs show preference for the HLA-DQ form of the molecule.
[5] Several staphylococcal SAgs are capable of cross-linking MHC molecules by binding to both the α and β chains.
[14] The SAg cross-links the MHC and the TCR inducing a signaling pathway that results in the proliferation of the cell and production of cytokines.
This occurs because a cognate antigen activates a T cell not because of its structure per se, but because its affinity allows it to bind the TCR for a lengthy enough time period, and the SAg mimics this temporal bonding.
[15] It is hypothesized that Fyn rather than Lck is activated by a tyrosine kinase, leading to the adaptive induction of anergy.
[17] This excessive uncoordinated release of cytokines, (especially TNF-α), overloads the body and results in rashes, fever, and can lead to multi-organ failure, coma and death.
The IL-4 and IL-10 downregulate production of IFN-gamma, MHC Class II, and costimulatory molecules on the surface of APCs.
MHC crosslinking also activates a signaling pathway that suppresses hematopoiesis and upregulates Fas-mediated apoptosis.
[22] If the initial inflammation is survived, the host cells become anergic or are deleted, resulting in a severely compromised immune system.
This effect is felt in cases of food poisoning, when SAg-producing bacteria release the toxin, which is highly resistant to heat.
This effect is due to the ability of SAgs to activate monocytic cells, stimulating the release of the cytokine TNF-α, leading to increased expression of adhesion molecules that recruit leukocytes to infected regions.
[22] The primary goals of medical treatment are to hemodynamically stabilize the patient and, if present, to eliminate the microbe that is producing the SAgs.
Synthetic antibodies and peptides have been created to mimic SAg-binding regions on the MHC class II, blocking the interaction and preventing T cell activation.
[15] The genes that regulate SAg expression also regulate mechanisms of immune evasion such as M protein and Bacterial capsule expression, supporting the hypothesis that SAg production evolved primarily as a mechanism of immune evasion.
[28] "Staphylococcal Superantigen-Like" (SSL) toxins are a group of secreted proteins structurally similar to SAgs.
Instead of binding to MHC and TCR, they target diverse components of innate immunity such as complement, Fc receptors, and myeloid cells.
[30] Minor lymphocyte stimulating (Mls; P03319) exotoxins were originally discovered in the thymic stromal cells of mice.
The virus manipulates the infected cell to express its own SAg genes, and this helps it to evade the host immune system.