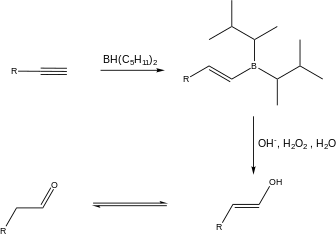
Syn and anti addition
In organic chemistry, syn- and anti-addition are different ways in which substituent molecules can be added to an alkene (R2C=CR2) or alkyne (RC≡CR).
The type of addition that occurs depends on multiple different factors of a reaction, and is defined by the final orientation of the substituents on the parent molecule.
Syn and anti addition are related to Markovnikov's rule for the orientation of a reaction, which refers to the bonding preference of different substituents for different carbons on an alkene or alkyne.
After addition to a straight-chain alkene such as ethene (C2H4), the resulting alkane will rapidly and freely rotate around its single sigma bond under normal conditions (i.e. room temperature).
However, if chirality or the specific absolute orientation of the substituents needs to be taken into account, knowing the type of addition is significant.