Targeted covalent inhibitors
[3] However, key changes in screening approaches, along with safety concerns, have made pharma reluctant to pursue covalent inhibitors in a systematic way (Liebler & Guengerich, 2005).
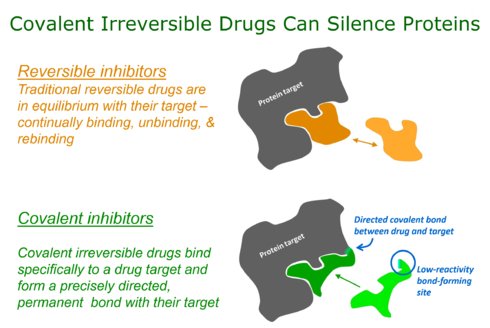
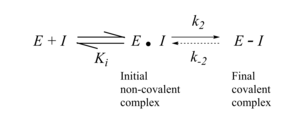
Covalent bonding thus allows high potency to be routinely achieved in compounds of low molecular mass, along with all the beneficial pharmaceutical properties that are associated with small size.
This has important and potentially advantageous consequences for drug pharmacodynamics in which the level and frequency of dosing relates to the extent and duration of the resulting pharmacological effect.
First, bioinformatics analysis is used to identify a nucleophilic amino acid (for example, cysteine) that is either inside or near to a functionally relevant binding site on a drug target, but is rare in that protein family.
Finally, structure-based computational methods are used to guide the design of modified ligands that have electrophilic functionality, and are positioned to react specifically with the nucleophilic amino acid in the target protein.
[1] Targeted covalent photoisomerizable ligands (photoswitches) have been developed to remotely and reversibly control the activity of receptor proteins with light.
[24] The pan-ErbB inhibitor Neratinib was approved in the US in 2017 and in the EU in 2018 for the extended adjuvant treatment of adult patients with early-stage HER2-overexpressed/amplified breast cancer after trastuzumab-based therapy.
[25][26] Ibrutinib, a covalent inhibitor of Bruton's tyrosine kinase, has been approved for the treatment of chronic lymphocytic leukemia, waldenstrom’s macroglobulinemia and mantle cell lymphoma.
It is in Phase III trials for the early treatment of SARS-CoV-2 infected patients who have not progressed to severe COVID-19 disease, and who do not immediately require hospitalisation.