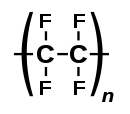
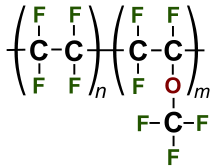
Polytetrafluoroethylene
PTFE and chemicals used in its production are some of the best-known and widely applied per- and polyfluoroalkyl substances (PFAS),[5] which are persistent organic pollutants.
[6][7] Dupont's spin-off Chemours today manufactures PTFE using an alternative chemical it calls GenX, another PFAS.
Although GenX was designed to be less persistent in the environment compared to PFOA, its effects may be equally harmful or even more detrimental than those of the chemical it has replaced.
John J. Beall (chemist), noticing a weight differential in his test cylinder, brought it to the attention of Roy Plunkett.
[12][13] By 1948, DuPont, which founded Kinetic Chemicals in partnership with General Motors, was producing over 910,000 kilograms (2,000,000 lb) of Teflon-brand polytetrafluoroethylene per year in Parkersburg, West Virginia.
He subsequently created the first PTFE-coated, non-stick pans under the brand name Tefal (combining "Tef" from "Teflon" and "al" from aluminium).
[22] The net equation is as follows: Because tetrafluoroethylene can explosively decompose to tetrafluoromethane (CF4) and carbon, a special apparatus is required for the polymerization to prevent hot spots that might initiate this dangerous side reaction.
[citation needed] PTFE's resistance to van der Waals forces means that it is the only known surface to which a gecko cannot stick.
[40][41] The viscosity and melting point can be decreased by inclusion of small amount of comonomers such as perfluoro (propylvinyl ether) and hexafluoropropylene (HFP).
[43] After a settling period, lasting from minutes to days, the mould is heated at 360 to 380 °C (680 to 716 °F),[43] allowing the fine particles to fuse (sinter) into a single mass.
[46][45] This application exploits the fact that PTFE has excellent dielectric properties, specifically low group velocity dispersion,[47] especially at high radio frequencies,[47] making it suitable for use as an excellent insulator in connector assemblies and cables, and in printed circuit boards used at microwave frequencies.
Combined with its high melting temperature, this makes PTFE the material of choice as a high-performance substitute for the weaker, higher dispersion and lower-melting-point polyethylene commonly used in low-cost applications.
[49] Its extremely high bulk resistivity makes it an ideal material for fabricating long-life electrets, the electrostatic analogues of permanent magnets.
The PTFE, used here as a film, prevents the non-production materials from sticking to the part being built, which is sticky due to the carbon-graphite or fiberglass plies being pre-pregnated with bismaleimide resin.
Because of its extreme non-reactivity and high temperature rating, PTFE is often used as the liner in hose assemblies, expansion joints, and in industrial pipe lines, particularly in applications using acids, alkalis, or other chemicals.
[53][54][55] PTFE is best known for its use in coating non-stick frying pans and other cookware, as it is hydrophobic and possesses fairly high heat resistance.
[75] Perfluorooctanoic acid (PFOA), a chemical formerly used in the manufacture of PTFE products such as non-stick coated cookware, can be carcinogenic for people who are exposed to it (see Ecotoxicity).
[76] Concerning levels of PFOA have been found in the blood of people who work in or live near factories where the chemical is used, and in people regularly exposed to PFOA-containing products such as some ski waxes and stain-resistant fabric coatings, but non-stick cookware was not found to be a major source of exposure, as the PFOA is burned off during the manufacturing process and not present in the finished product.
[79] PFOA has been detected in the blood of many individuals of the general US population in the low and sub-parts per billion range, and levels are higher in chemical plant employees and surrounding subpopulations.
[86][87] However, the EPA has classified GenX as more toxic than PFOA[8] and it has proven to be a "regrettable substitute";[88] its effects may be equally harmful or even more detrimental than those of the chemical it was meant to replace.
[89] Fayetteville Works was the site where DuPont began manufacture of PFOA after the lawsuit in Parkersburg WV halted their production there.
In June of 2017, The Wilmington Star-News broke the story[90] that GenX was found in the Cape Fear River – the drinking water supply for 500,000 people.
The North Carolina Department of Environmental Quality (NC DEQ) records[91] indicate that DuPont started release PFAS into the area beginning in 1976 with the production of Nafion, and that PFAS including GenX had been released as a byproduct of the production of Vinyl Ethers since 1980, exposing the Cape Fear Basin for decades.
The result was a Consent Order,[92] signed February 25, 2019 by Cape Fear River Watch, NC DEQ, and Chemours.
[94] The Teflon trade name is also used for other polymers with similar compositions: These retain the useful PTFE properties of low friction and nonreactivity, but are also more easily formable.