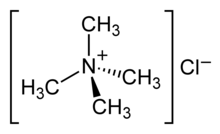
Tetramethylammonium chloride
Tetramethylammonium chloride is one of the simplest quaternary ammonium salts, with four methyl groups tetrahedrally attached to the central N. The chemical formula (CH3)4N+Cl− is often abbreviated further as Me4N+Cl−.
[2] In the laboratory, it has fewer synthetic chemical applications than quaternary ammonium salts containing longer N-alkyl substituents, which are used extensively as phase-transfer catalysts.
[3] It is produced by the alkylation of ammonium chloride with dimethyl carbonate in the presence of an ionic liquid catalyst.
[4] Except under extraordinary conditions,[5] it is typically employed as a source of the inert counter cation Me4N+.
[8] Diverse data on human exposure, environmental toxicology and environmentally-related chemistry is available through the NIH Toxnet database.