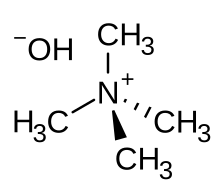
Tetramethylammonium hydroxide
Although TMAH has virtually no odor when pure, samples often have a strong fishy smell due to presence of trimethylamine which is a common impurity.
This report also provides details for isolation of TMAH as its pentahydrate, noting the existence of a trihydrate, and emphasizes the avidity which even the former exhibits for atmospheric moisture and carbon dioxide.
[5] TMAH undergoes simple acid-base reactions to produce tetramethylammonium (TMA) salts whose anion is derived from the acid used.
Illustrative is the preparation of tetramethylammonium fluoride:[6] TMAH and many other TMA salts containing simple anions thermally decompose into trimethylamine.
[12] TMAH belongs to the family of quaternary ammonium hydroxide (QAH) solutions and is commonly used to anisotropically etch silicon.
TMAH is preferred over sodium or potassium hydroxide in applications that are sensitive to metal ion contamination.
The etch rate for silicon dioxide in TMAH varies with the quality of the film, but is generally on the order of 0.1 nm/minute.
[11] The tetramethylammonium ion [14] affects nerves and muscles, causing difficulties in breathing, muscular paralysis and possibly death.