Thermodynamic process
This is a theoretical exercise in differential geometry, as opposed to a description of an actually possible physical process; in this idealized case, the calculation may be exact.
The quantities of primary concern describe the states of the inflow and the outflow materials, and, on the side, the transfers of heat, work, and kinetic and potential energies for the vessel.
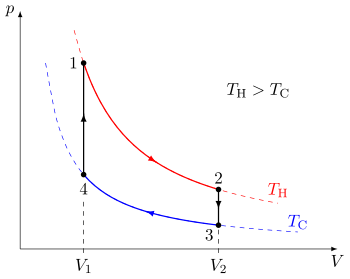
A cycle is a sequence of a small number of thermodynamic processes that indefinitely often, repeatedly returns the system to its original state.
If, however, the several staged processes are idealized and quasi-static, then the cycle is described by a path through a continuous progression of equilibrium states.
The quantities of primary concern describe the states of the inflow and the outflow materials, and, on the side, the transfers of heat, work, and kinetic and potential energies for the vessel.
A quasi-static thermodynamic process can be visualized by graphically plotting the path of idealized changes to the system's state variables.
This equation can be used to accurately characterize processes of certain systems, notably the compression or expansion of a gas, but in some cases, liquids and solids.
According to Planck, one may think of three main classes of thermodynamic process: natural, fictively reversible, and impossible or unnatural.
For thermodynamics, a natural process is a transfer between systems that increases the sum of their entropies, and is irreversible.
[2] Natural processes may occur spontaneously upon the removal of a constraint, or upon some other thermodynamic operation, or may be triggered in a metastable or unstable system, as for example in the condensation of a supersaturated vapour.
[4] Planck emphasised the occurrence of friction as an important characteristic of natural thermodynamic processes that involve transfer of matter or energy between system and surroundings.
To describe the geometry of graphical surfaces that illustrate equilibrium relations between thermodynamic functions of state, no one can fictively think of so-called "reversible processes".
It may be imagined as happening infinitely slowly so that the system passes through a continuum of states that are infinitesimally close to equilibrium.