Rankine cycle
Heat energy is supplied to the system via a boiler where the working fluid (typically water) is converted to a high-pressure gaseous state (steam) in order to turn a turbine.
After passing over the turbine the fluid is allowed to condense back into a liquid state as waste heat energy is rejected before being returned to boiler, completing the cycle.
Possible heat sources include combustion of fossil fuels such as coal, natural gas, and oil, use of mined resources for nuclear fission, renewable fuels like biomass and ethanol, and energy capture of natural sources such as concentrated solar power and geothermal energy.
Common heat sinks include ambient air above or around a facility and bodies of water such as rivers, ponds, and oceans.
The greater the differential, the more mechanical power can be efficiently extracted out of heat energy, as per Carnot's theorem.
The idea is that very hot combustion products are first expanded in a gas turbine, and then the exhaust gases, which are still relatively hot, are used as a heat source for the Rankine cycle, thus reducing the temperature difference between the heat source and the working fluid and therefore reducing the amount of entropy generated by irreversibility.
Rankine engines generally operate in a closed loop in which the working fluid is reused.
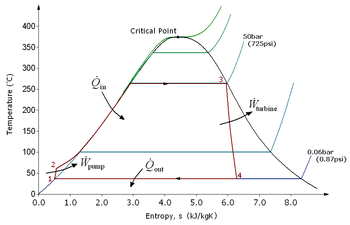
This "exhaust" heat is represented by the "Qout" flowing out of the lower side of the cycle shown in the T–s diagram below.
While many substances can be used as the working fluid, water is usually chosen for its simple chemistry, relative abundance, low cost, and thermodynamic properties.
The Rankine cycle shown here prevents the state of the working fluid from ending up in the superheated vapor region after the expansion in the turbine, [1] which reduces the energy removed by the condensers.
The actual vapor power cycle differs from the ideal Rankine cycle because of irreversibilities in the inherent components caused by fluid friction and heat loss to the surroundings; fluid friction causes pressure drops in the boiler, the condenser, and the piping between the components, and as a result the steam leaves the boiler at a lower pressure; heat loss reduces the net work output, thus heat addition to the steam in the boiler is required to maintain the same level of net work output.
defines the thermodynamic efficiency of the cycle as the ratio of net power output to heat input.
The purpose of a reheating cycle is to remove the moisture carried by the steam at the final stages of the expansion process.
Another variation sends bleed steam from between turbine stages to feedwater heaters to preheat the water on its way from the condenser to the boiler.
Alternatively, fluids can be used that have boiling points above water, and this may have thermodynamic benefits (See, for example, mercury vapour turbine).