Thin-film solar cell
Other commercial applications use rigid thin film solar panels (interleaved between two panes of glass) in some of the world's largest photovoltaic power stations.
Still, many thin-film technologies have been found to have shorter operational lifetimes and larger degradation rates than first-generation cells in accelerated life testing, which has contributed to their somewhat limited deployment.
In 1970, Zhores Alferov's team at Ioffe Institute created the first gallium arsenide (GaAs) solar cells, later winning the 2000 Nobel prize in Physics for this and other work.
[12] Only seven years later in 1999, the U.S. National Renewable Energy Laboratory (NREL) and Spectrolab collaborated on a three-junction gallium arsenide solar cell that reached 32% efficiency.
[13] That same year, Kiss + Cathcart designed transparent thin-film solar cells for some of the windows in 4 Times Square, generating enough electricity to power 5-7 houses.
[21] In 2016, Vladimir Bulović's Organic and Nanostructured Electronics (ONE) Lab at the Massachusetts Institute of Technology (MIT) created thin-film cells light enough to sit on top of soap bubbles.
[33]: 31 CdTe also performs better than most other thin-film PV materials across many important environmental impact factors like global warming potential and heavy metal emissions.
[35] Although the toxicity of cadmium may not be that much of an issue and environmental concerns completely resolved with the recycling of CdTe modules at the end of their life time,[36] there are still uncertainties[37] and the public opinion is skeptical towards this technology.
This is called the Staebler-Wronski effect (SWE) – a typical loss in electrical output due to changes in photoconductivity and dark conductivity caused by prolonged exposure to sunlight.
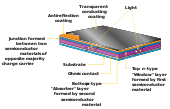
Although this degradation is perfectly reversible upon annealing at or above 150 °C, conventional c-Si solar cells do not exhibit this effect in the first place.Its basic electronic structure is the p-i-n junction.
The amorphous structure of a-Si implies high inherent disorder and dangling bonds, making it a bad conductor for charge carriers.
Significant research has been invested into these technologies as they promise to achieve the goal of producing low-cost, high-efficiency solar cells with smaller environmental impacts.
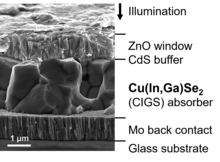
Copper zinc tin sulfide or Cu(Zn,Sn)(S,Se)2, commonly abbreviated CZTS, and its derivatives CZTSe and CZTSSe belong to a group chalcogenides (like CdTe and CIGS/CIS) sometimes called kesterites.
Instead, they are constructed using a layer of photoactive dye mixed with semiconductor transition metal oxide nanoparticles on top of a liquid electrolyte solution, surrounded by electrical contacts made of platinum or sometimes graphene and encapsulated in glass.
[68] This is mostly due to their chemical instability when exposed to light, moisture, UV radiation, and high temperatures which may even cause them to undergo a structural transition that impacts the operation of the device.
However, QDPV cells tend to have high environmental impacts compared to other thin-film PV materials, especially human toxicity and heavy metal emissions.
[34] In 2022, semitransparent solar cells that are as large as windows were reported,[72] after team members of the study achieved record efficiency with high transparency in 2020.
[citation needed] Concentrator photovoltaics use an optical system of lenses that sit on top of the cell to focus light from a larger area onto the device, similar to a funnel for sunlight.
[91][92] Besides surface texturing, the plasmonic light-trapping scheme attracted a lot of attention to aid photocurrent enhancement in thin film solar cells.
[93][94] This method makes use of collective oscillation of excited free electrons in noble metal nanoparticles, which are influenced by particle shape, size and dielectric properties of the surrounding medium.
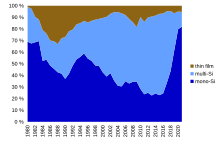
[3] With the advances in conventional crystalline silicon (c-Si) technology in recent years, and the falling cost of the polysilicon feedstock, that followed after a period of severe global shortage, pressure increased on manufacturers of commercial thin-film technologies, including amorphous thin-film silicon (a-Si), cadmium telluride (CdTe), and copper indium gallium diselenide (CIGS), leading to the bankruptcy of several companies.
[102] In 2014, Korean LG Electronics terminated research on CIGS restructuring its solar business, and Samsung SDI decided to cease CIGS-production, while Chinese PV manufacturer Hanergy is expected to ramp up production capacity of their 15.5% efficient, 650 mm×1650 mm CIGS-modules.
[119][120] In 1998, scientists at the National Renewable Energy Laboratory (NREL) predicted that production of thin-film PV systems at a cost of $50 per m2 could someday be possible, which would make them extremely economically viable.
Beyond key factors like greenhouse gas (GHG) emissions, questions have been raised about the environmental and health impacts of potentially toxic materials like cadmium that are used in many solar cell technologies.
[63] Low realized efficiencies are also the driving factor behind the relatively large GWP of quantum dot solar cells, despite the potential for these materials to exhibit multiple exciton generation (MEG) from a single photon.
This is significantly better than the 60% reduction compared to mono crystalline silicon currently realized, and therefore improving OPV cell lifetimes is a priority for decreasing overall environmental impact.
Perovskite solar cells (not included in the chart) typically have significantly larger global warming potential than other thin-film materials in cradle-to-grave LCA, around 5-8x worse than mono crystalline silicon at 150g CO2-eq /kWh.
The application in which the modules are used and the recycling process (if any) for the materials can also play a large role in the overall energy efficiency and greenhouse gas emissions over the lifetime of the cell.
[128] Cadmium is a highly hazardous material[129] that causes kidney, bone, and lung damage and is thought to increase the risk of developing cancer.
[127] Feedstock Cd presents a larger risk, as do precursor materials like CdS, and cadmium acetate, which are frequently used in other photovoltaic cells as well, and often contribute significantly to environmental impact factors such as human toxicity and heavy metal emission.