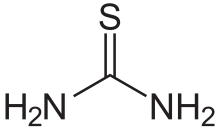
Thiourea
Thiourea is manufactured by the reaction of hydrogen sulfide with calcium cyanamide in the presence of carbon dioxide.
It is mainly consumed as a precursor to thiourea dioxide, which is a common reducing agent in textile processing.
[6] Recently thiourea has been investigated for its multiple desirable properties as a fertilizer especially under conditions of environmental stress.
[7] It may be applied in various capacities, such as a seed pretreatment (for priming), foliar spray or medium supplementation.
Other industrial uses of thiourea include production of flame retardant resins, and vulcanization accelerators.
[14] Dimethyl sulfide is also an effective reagent for this reaction, but it is highly volatile (boiling point 37 °C) and has an obnoxious odor whereas thiourea is odorless and conveniently non-volatile (reflecting its polarity).
[22] A lixiviant for gold and silver leaching can be created by selectively oxidizing thiourea, bypassing the steps of cyanide use and smelting.
[23] Thiourea is an essential reagent in the Kurnakov test used to differentiate cis- and trans- isomers of certain square planar platinum complexes.
The reaction was discovered in 1893 by Russian chemist Nikolai Kurnakov and is still performed as an assay for compounds of this type.
[25] A goitrogenic effect (enlargement of the thyroid gland) has been reported for chronic exposure, reflecting the ability of thiourea to interfere with iodide uptake.