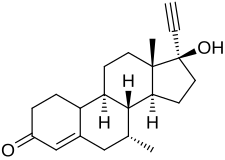
Tibolone
Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis.
[12][19] Tibolone is used in the treatment of menopausal symptoms like hot flashes and vaginal atrophy, postmenopausal osteoporosis, and endometriosis.
[25][22][26] The medication may have greater benefits on libido than standard menopausal hormone therapy, which may be related to its androgenic effects.
[27] The decrease in risk was greater than that observed with most of the aromatase inhibitors and selective estrogen receptor modulators that were included in the analysis.
[27] However, paradoxically, other research has found evidence supporting an increased risk of breast cancer with tibolone.
[30] It is typically used once daily at a dosage of 1.25 or 2.5 mg.[30] A report in September 2009 from Health and Human Services' Agency for Healthcare Research and Quality suggests that tamoxifen, raloxifene, and tibolone used to reduce the risk of breast cancer significantly reduce the occurrence of invasive breast cancer in midlife and older women, but also increase the risk of adverse effects.
[31] Tibolone can infrequently produce androgenic side effects such as acne and increased facial hair growth.
[38][39][40][41][42][43] By 2008, these researchers had asserted that tibolone is not aromatized in women and that the previous findings of 7α-methylethinylestradiol detection were merely a methodological artifact.
[40][42][43] In accordance, a 2009 study found that an aromatase inhibitor had no effect on the estrogenic potencies of tibolone or its metabolites in vitro, unlike the case of testosterone.
[44] These findings are also in accordance with the fact that tibolone decreases sex hormone-binding globulin (SHBG) levels by 50% in women and does not increase the risk of venous thromboembolism (VTE) (RRTooltip Rate ratio = 0.92), which would not be expected if the medication formed a potent, liver metabolism-resistant estrogen similar to ethinylestradiol in important quantities.
[48][49] They have claimed that 19-nortestosterone derivatives like tibolone, due to lacking a C19 methyl group, indeed are not substrates of the classical aromatase enzyme, but instead are still transformed into the corresponding estrogens by other cytochrome P450 monooxygenases.
[41][48][49] In accordance, the closely structurally related AAS trestolone (7α-methyl-19-nortestosterone or 17α-desethynyl-δ4-tibolone) has been found to be transformed into 7α-methylestradiol by human placental microsomes in vitro.
[1][49] In spite of its high affinity for the PR however, δ4-tibolone possesses only weak progestogenic activity, about 13% of that of norethisterone.
[49][1] Indeed, the androgenic effects of tibolone have been ranked as stronger than those of all other commonly used 19-nortestosterone progestins (e.g., norethisterone, levonorgestrel, others).
[49][1] The androgenic effects of tibolone have been postulated to be involved in the reduced breast cell proliferation, reduced breast cancer risk, improvement in sexual function, less unfavorable changes in hemostatic parameters relative to estrogen–progestogen combinations, and changes in liver protein synthesis (e.g., 30% reductions in HDL cholesterol levels, 20% reduction in triglyceride levels, and 50% reduction in SHBG levels) observed with tibolone.
[49][1] They are also responsible for the androgenic side effects of tibolone such as acne and increased hair growth in some women.
[6] However, their affinities for these receptors are low, and tibolone has been described as possessing no clinically significant glucocorticoid, antiglucocorticoid, mineralocorticoid, or antimineralocorticoid activity.