Lovastatin
Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease.
[9] This can be life-threatening if not recognised and treated in time, so any unexplained muscle pain or weakness whilst on lovastatin should be promptly mentioned to the prescribing doctor.
[12] As with atorvastatin, simvastatin, and other statin drugs metabolized via CYP3A4, drinking grapefruit juice during lovastatin therapy may increase the risk of side effects.
[17] It is likely that these effect are mediated by the properties of statins to reduce proteasome activity, leading to an accumulation of cyclin-dependent kinase inhibitors p21 and p27, and to subsequent G1-phase arrest, as seen in cells of different cancer lines.
[18][19] Compactin and lovastatin, natural products with a powerful inhibitory effect on HMG-CoA reductase, were discovered in the 1970s, and taken into clinical development as potential drugs for lowering LDL cholesterol.
[21][22] In 1982, some small-scale clinical investigations of lovastatin, a polyketide-derived natural product isolated from Aspergillus terreus, in very high-risk patients were undertaken, in which dramatic reductions in LDL cholesterol were observed, with very few adverse effects.
[37] In 1998, the FDA placed a ban on the sale of dietary supplements derived from red yeast rice, which naturally contains lovastatin, arguing that products containing prescription agents require drug approval.
Cholesterol is biosynthesized in a series of more than 25 separate enzymatic reactions that initially involves three successive condensations of acetyl-CoA units to form the six-carbon compound 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA).
A major rate-limiting step in the pathway is at the level of the microsomal enzyme that catalyzes the conversion of HMG CoA to mevalonic acid, and that has been considered to be a prime target for pharmacologic intervention for several years.
Inhibition of this enzyme could lead to accumulation of HMG CoA, a water-soluble intermediate that is, then, capable of being readily metabolized to simpler molecules.
The first breakthrough in efforts to find a potent, specific, competitive inhibitor of HMG CoA reductase occurred in 1976, when Endo et al. reported the discovery of mevastatin, a highly functionalized fungal metabolite, isolated from cultures of Penicillium citrium.
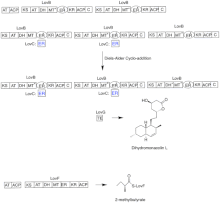
In a parallel pathway, the diketide side chain of lovastatin is synthesized by another highly reducing type I polyketide synthase enzyme encoded by LovF .
Lastly, the side chain, 2-methylbutyrate (M) is covalently attached to C-8 hydroxy group of monacolin J (L) by a transesterase encoded by LovD to form lovastatin.
Lovastatin is a naturally occurring compound found in low concentrations in food such as oyster mushrooms,[45] red yeast rice,[46] and Pu-erh.
[47] Mevacor, Advicor (as a combination with niacin), Altocor, Altoprev[citation needed] In plant physiology, lovastatin has occasionally been used as inhibitor of cytokinin biosynthesis.