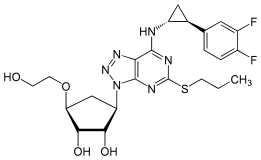
Ticagrelor
The most common side effects include dyspnea (difficulty breathing), bleeding and raised uric acid level in the blood.
[17][18][19] People who develop tolerable dyspnoea as a side effect of ticagrelor should be reassured to continue therapy, as it does not impact on the drug's cardiovascular benefit and bleeding risk in acute coronary syndrome (ACS).
[18][21] Ventricular pauses ≥3 seconds may occur in people with ACS the first week of treatment, but are likely to be mostly asymptomatic and transient, without causing increased clinical bradycardic adverse events.
[24] Inhibitors of the liver enzyme CYP3A4, such as ketoconazole and possibly grapefruit juice, increase blood plasma levels of ticagrelor and consequently can lead to bleeding and other adverse effects.
In contrast to the other antiplatelet drugs, ticagrelor has a binding site different from ADP, making it an allosteric antagonist, and the blockage is reversible.
[8] The PLATO trial concluded superiority of ticagrelor compared to clopidogrel in reducing the rate of death from vascular causes, MI, and stroke in people presenting with acute coronary syndromes.
[14] A post-hoc subgroup analysis of the PLATO trial suggested a reduction in total mortality with ticagrelor compared to clopidogrel in people with non-ST elevation acute coronary syndrome.
[38] The PLATO trial[39] found that ticagrelor use, in conjunction with low-dose aspirin (where tolerated), had better all-cause mortality rates than the same treatment plan with clopidogrel (4.5% vs. 5.9%) in treating people with acute coronary syndrome.
[41][42][43] The guidelines recommend that people of East Asian origin exercise caution and that treatment continuation after six months be based on net-clinical benefit.
[45] A 2019 study showed antibacterial activity against antibiotic-resistant Gram-positive bacteria including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.
[46] This study used concentrations of ticagrelor for bactericidal activity that far exceeded those achieved by standard post acute coronary syndrome doses.