Topical gels
[1][2][5][3][8] A gel refers to the semi- solid, 3-dimensional matrix formed from an interspersed system of colloidal particles or the permeation of a solvent into an entwined polymer chain network.
[1][2][5][3][8] Pharmaceutical gels are formed by adding a gelator (gelling agent) to the solvent [5][6] and active ingredient mixture.
Gelators used in gel formulation can be small molecules with low molecular weight or polymers (synthetic, semi-synthetic or natural).
Gels have certain special properties that put them apart from other dosage forms, in terms of swelling, syneresis, ageing, rigidity and rheology.
[5] Some manufacturers decide to use organogels as a medium for drug delivery due to its potentially emollient effect.
[1][6][10] This emollient effect is particularly helpful in formulation of topical gels for patients with dry and irritated skin.
[1][6][9] Formulation of topical gels is determined by important factors such as appearance, odor, spreadability, extrudability, viscosity, pH, texture, microbial contamination potential and bioavailability.
[3] The ingredients in topical gel formulation can be broadly categorized into four types: gelator, solvent, drug, and excipients.
[8] There are many types of gelators, of which carbomers are more frequently used due to their ability to thicken gels across a wide range of pH.
[5] Some examples of solvents include purified water,[3] glycerin, glycols, alcohols, sucrose, toluene, and mineral oils.
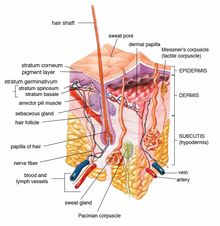
[5][10] Topical delivery is often used for drugs that are easily degraded in the GI tract, or are highly susceptible to hepatic first pass effect.
[1][9] There are a number of physicochemical and biological properties that determine whether a drug is suitable for being delivered topically through a gel dosage form.
[1][6] Many classes of excipients can be used as penetration enhancers, such as glycerin, sulfoxides and related analogues, pyrrolidines, fatty acid and ethanol, surfactants etc.
[5][1][2][8] Topical gels are commonly used in cosmetics, which include shampoos, dentifrices, skin and hair care formulations and fragrance products,[1][2] and can be used to treat scalp inflammation.
[6] The texture of topical gels is less greasy as it contains a higher proportion of water compared with cream and ointment.
[3][1][2][8] These gels have an excellent spreading property and cooling effect due to solvent evaporation, and also has a higher retention time on the skin.
[1][2] The formulation and manufacturing processes of topical gels are relatively simpler and more cost effective than other semisolid dosage forms.
[5][1][8] The release profile of the gel can be modified by altering the properties of the gelator, allowing for continuous drug delivery.
[5][8] The drug can penetrate deeply into the skin [2] and be directly delivered to the target site, as the topical application allows it to avoid hepatic first pass metabolism.
[1][2][8][6] Difficulties in gastrointestinal absorption caused by pH, enzymatic activity and drug-food interactions can be minimized, while at the same time avoiding GI irritation.
[5][8] The rheology of some gels are easily altered by environmental factors such as temperature and humidity,[5] resulting in stricter storage requirements.
Considering the direct route of administration, drugs must be very small in size to have an effective plasma concentration for action.
The particle size and other properties of the drug may also affect its absorption through the skin barrier,[2] resulting in an unreliable effect.