Steroid
This is an accepted version of this page A steroid is an organic compound with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration.
Examples include the lipid cholesterol, sex hormones estradiol and testosterone,[2]: 10–19 anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone.
Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings.
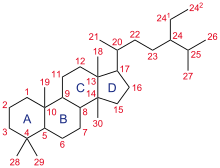
Gonane, also known as steran or cyclopentanoperhydrophenanthrene, the nucleus of all steroids and sterols,[11][12] is composed of seventeen carbon atoms in carbon-carbon bonds forming four fused rings in a three-dimensional shape.
When the two methyl groups and eight carbon side chains (at C-17, as shown for cholesterol) are present, the steroid is said to have a cholestane framework.
[13] Examples of steroid structures are: In addition to the ring scissions (cleavages), expansions and contractions (cleavage and reclosing to a larger or smaller rings)—all variations in the carbon-carbon bond framework—steroids can also vary: For instance, sterols such as cholesterol and lanosterol have a hydroxyl group attached at position C-3, while testosterone and progesterone have a carbonyl (oxo substituent) at C-3.
Almost all biologically relevant steroids can be presented as a derivative of a parent cholesterol-like hydrocarbon structure that serves as a skeleton.
The derivatives carry various functional groups called suffixes or prefixes after the respective numbers, indicating their position in the steroid nucleus.
[16] There are widely used trivial steroid names of natural origin with significant biologic activity, such as progesterone, testosterone or cortisol.
[14] Unsaturated carbons (generally, ones that are part of a double bond) in the steroid nucleus are indicated by changing -ane to -ene.
[23] This change was traditionally done in the parent name, adding a prefix to denote the position, with or without Δ (Greek capital delta) which designates unsaturation, for example, 4-pregnene-11β,17α-diol-3,20-dione (also Δ4-pregnene-11β,17α-diol-3,20-dione) or 4-androstene-3,11,17-trione (also Δ4-androstene-3,11,17-trione).
However, the Nomenclature of Steroids recommends the locant of a double bond to be always adjacent to the syllable designating the unsaturation, therefore, having it as a suffix rather than a prefix, and without the use of the Δ character, i.e. pregn-4-ene-11β,17α-diol-3,20-dione or androst-4-ene-3,11,17-trione.
According to the rule set in the Nomenclature of Steroids, the terminal "e" in the parent structure name should be elided before the vowel (the presence or absence of a number does not affect such elision).
[37][38] Eukaryotic cells, encompassing animals, plants, fungi, and protists, are characterized by their complex cellular structures, including a true nucleus and membrane-bound organelles.
[39] Sterols, a subgroup of steroids, play crucial roles in maintaining membrane fluidity, supporting cell signaling, and enhancing stress tolerance.
[42] Prokaryotic sterol synthesis involves the tetracyclic steroid framework, as found in myxobacteria,[43] as well as hopanoids, pentacyclic lipids that regulate bacterial membrane functions.
[47] All mushrooms contain large quantities of ergosterol, in the range of tens to hundreds of milligrams per 100 grams of dry weight.
[47] However, not all fungi utilize ergosterol in their cellular membranes; for example, the pathogenic fungal species Pneumocystis jirovecii does not, which has important clinical implications (given the mechanism of action of many antifungal drugs).
Examples of this classification include: In biology, it is common to name the above steroid classes by the number of carbon atoms present when referring to hormones: C18-steroids for the estranes (mostly estrogens), C19-steroids for the androstanes (mostly androgens), and C21-steroids for the pregnanes (mostly corticosteroids).
For instance, the prototypical secosteroid cholecalciferol, vitamin D3 (shown), is in the 9,10-secosteroid subclass and derives from the cleavage of carbon atoms C-9 and C-10 of the steroid B-ring; 5,6-secosteroids and 13,14-steroids are similar.
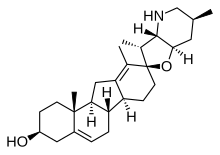
For instance, ewes who graze on corn lily ingest cyclopamine (shown) and veratramine, two of a sub-family of steroids where the C- and D-rings are contracted and expanded respectively via a biosynthetic migration of the original C-13 atom.
Ingestion of these C-nor-D-homosteroids results in birth defects in lambs: cyclopia from cyclopamine and leg deformity from veratramine.
Subsequent epoxidation and cyclization of squalene generate lanosterol, which is the starting point for additional modifications into other steroids (steroidogenesis).
Two classes of drugs target the mevalonate pathway: statins (like rosuvastatin), which are used to reduce elevated cholesterol levels,[70] and bisphosphonates (like zoledronate), which are used to treat a number of bone-degenerative diseases.
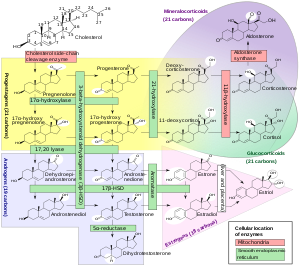
The major classes of steroid hormones, as noted above (with their prominent members and functions), are the progestogens, corticosteroids (corticoids), androgens, and estrogens.
These reactions introduce oxygen into the steroid ring, allowing the cholesterol to be broken up by other enzymes into bile acids.
[87][page needed] The methods of isolation to achieve the two scales of product are distinct, but include extraction, precipitation, adsorption, chromatography, and crystallization.
In both cases, the isolated substance is purified to chemical homogeneity; combined separation and analytical methods, such as LC-MS, are chosen to be "orthogonal"—achieving their separations based on distinct modes of interaction between substance and isolating matrix—to detect a single species in the pure sample.
[88][89][90] The addition and modification of functional groups is key when producing the wide variety of medications available within this chemical classification.
[93] The efforts of Syntex, a company involved in the Mexican barbasco trade, used Dioscorea mexicana to produce the sapogenin diosgenin in the early days of the synthetic steroid pharmaceutical industry.