Triglyceride
A triglyceride (from tri- and glyceride; also TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids.
Unsaturated fats tend to have a lower melting point than saturated analogues; as a result, they are often liquid at room temperature.
As a result, ruminant animal fat contains odd-numbered fatty acids, such as 15, due to the action of bacteria in the rumen.
Cocoa butter is unusual in that it is composed of only a few triglycerides, derived from palmitic, oleic, and stearic acids in the 1-, 2-, and 3-positions of glycerol, respectively.
[citation needed] Triglycerides are tri-esters derived from the condensation reaction of glycerol with three fatty acids.
[4] An early step in the biosynthesis is the formation of the glycerol-1-phosphate:[4] The three oxygen atoms in this phosphate ester are differentiated, setting the stage for regiospecific formation of triglycerides, as the diol reacts selectively with coenzyme-A derivatives of the fatty acids, RC(O)S–CoA: The phosphate ester linkage is then hydrolysed to make way for the introduction of a third fatty acid ester: Fats are often named after their source, e.g., olive oil, cod liver oil, shea butter, tail fat.
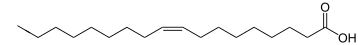
For fatty acids, for example, the position and orientation of carbon-carbon double bonds is specified counting from the carboxyl functional group.
[citation needed] For human nutrition, an important classification of fats is based on the number and position of double bonds in the constituent fatty acids.
(The names refer to the fact that each double bond means two fewer hydrogen atoms in the chemical formula.
)[10][11] Unsaturated fatty acids are further classified into monounsaturated (MUFAs), with a single double bond, and polyunsaturated (PUFAs), with two or more.
[12]) Saturated fats generally have a higher melting point than unsaturated ones with the same molecular weight, and thus are more likely to be solid at room temperature.
For example, the animal fats tallow and lard are high in saturated fatty acid content and are solids.
The heats of combustion of saturated, mono-, di-, and tri-unsaturated 18-carbon fatty acid esters have been measured as 2859, 2828, 2794, and 2750 kcal/mol, respectively; or, on a weight basis, 10.75, 10.71, 10.66, and 10.58 kcal/g – a decrease of about 0.6% for each additional double bond.
While it is the nutritional aspects of polyunsaturated fatty acids that are generally of greatest interest, these materials also have non-food applications.
Linseed oil is rich in di- and tri-unsaturated fatty acid components, which tend to harden in the presence of oxygen.