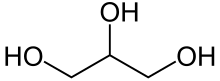
Glycerol
During World War II, synthetic glycerol processes became a national defense priority because it is a precursor to nitroglycerine.
The U.S. Food and Drug Administration (FDA) carbohydrate designation includes all caloric macronutrients excluding protein and fat.
Glycerol has a caloric density similar to table sugar, but a lower glycemic index and different metabolic pathway within the body.
[17] Glycerol is used in medical, pharmaceutical and personal care preparations, often as a means of improving smoothness, providing lubrication, and as a humectant.
[23] Taken orally (often mixed with fruit juice to reduce its sweet taste), glycerol can cause a rapid, temporary decrease in the internal pressure of the eye.
[24] In 2017, researchers showed that the probiotic Limosilactobacillus reuteri bacteria can be supplemented with glycerol to enhance its production of antimicrobial substances in the human gut.
[26] The glycerol content acts to add viscosity to the bio-ink without adding large protein, saccharide, or glycoprotein molecules.
Glycerol was historically used as an anti-freeze for automotive applications before being replaced by ethylene glycol, which has a lower freezing point.
It is also used as a cryoprotectant where the glycerol is dissolved in water to reduce damage by ice crystals to laboratory organisms that are stored in frozen solutions, such as fungi, bacteria, nematodes, and mammalian embryos.
Nitroglycerin under the name glyceryl trinitrate (GTN) is commonly used to relieve angina pectoris, taken in the form of sub-lingual tablets, patches, or as an aerosol spray.
[41] Glycerol is used by set decorators when filming scenes involving water to prevent an area meant to look wet from drying out too quickly.
Blood glycerol levels in diabetic patients average three times higher than healthy controls.
Direct glycerol treatment of testes has been found to cause significant long-term reduction in sperm count.
In some organisms, the glycerol component can enter the glycolysis pathway directly and, thus, provide energy for cellular metabolism (or, potentially, be converted to glucose through gluconeogenesis).
Before glycerol can enter the pathway of glycolysis or gluconeogenesis (depending on physiological conditions), it must be converted to their intermediate glyceraldehyde 3-phosphate in the following steps: GlycerolGlycerol kinaseGlycerol-3-phosphateGlycerol-3-phosphate dehydrogenaseDihydroxyacetone phosphateTriosephosphate isomeraseGlyceraldehyde 3-phosphateThe enzyme glycerol kinase is present mainly in the liver and kidneys, but also in other body tissues, including muscle and brain.
It does not appear to cause toxicity when inhaled, although changes in cell maturity occurred in small sections of lung in animals under the highest dose measured.
A sub-chronic 90-day nose-only inhalation study in Sprague–Dawley (SD) rats exposed to 0.03, 0.16 and 0.66 mg/L glycerin (Per liter of air) for 6-hour continuous sessions revealed no treatment-related toxicity other than minimal metaplasia of the epithelium lining at the base of the epiglottis in rats exposed to 0.66 mg/L glycerin.
While intoxication as a result of excessive glycerol consumption is rare and its symptoms generally mild, occasional reports of hospitalization have occurred.
[67] Food Standards Scotland advises that slush ice drinks containing glycerol should not be given to children under the age of 4, owing to the risk of intoxication.
[69] This followed an occurrence of hundreds of fatal poisonings in Panama resulting from a falsified import customs declaration by Panamanian import/export firm Aduanas Javier de Gracia Express, S. A.
[70][71] Between 1990 and 1998, incidents of DEG poisoning reportedly occurred in Argentina, Bangladesh, India, and Nigeria, and resulted in hundreds of deaths.
In 1937, more than one hundred people died in the United States after ingesting DEG-contaminated elixir sulfanilamide, a drug used to treat infections.
[72] The origin of the gly- and glu- prefixes for glycols and sugars is from Ancient Greek γλυκύς glukus which means sweet.
1811 by Michel Eugène Chevreul to denote what was previously called "sweet principle of fat" by its discoverer Carl Wilhelm Scheele.