Ultraviolet–visible spectroscopy
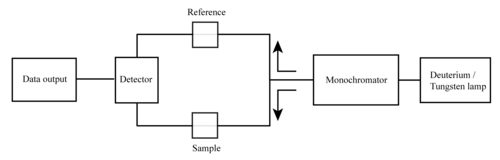
[4][5] A UV-Vis spectrophotometer is an analytical instrument that measures the amount of ultraviolet (UV) and visible light that is absorbed by a sample.
It is a widely used technique in chemistry, biochemistry, and other fields, to identify and quantify compounds in a variety of samples.
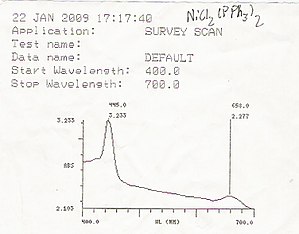
Transition metal complexes are often colored (i.e., absorb visible light) owing to the presence of multiple electronic states associated with incompletely filled d orbitals.
The Woodward–Fieser rules, for instance, are a set of empirical observations used to predict λmax, the wavelength of the most intense UV-Vis absorption, for conjugated organic compounds such as dienes and ketones.
The nature of the solvent, the pH of the solution, temperature, high electrolyte concentrations, and the presence of interfering substances can influence the absorption spectrum.
To apply UV-Vis spectroscopy to analysis, these variables must be controlled or accounted for in order to identify the substances present.
The Beer–Lambert law is useful for characterizing many compounds but does not hold as a universal relationship for the concentration and absorption of all substances.
A 2nd order polynomial relationship between absorption and concentration is sometimes encountered[13] for very large, complex molecules such as organic dyes (xylenol orange or neutral red, for example).
[14][15] UV–Vis spectroscopy is also used in the semiconductor industry to measure the thickness and optical properties of thin films on a wafer.
UV–Vis spectrometers are used to measure the reflectance of light, and can be analyzed via the Forouhi–Bloomer dispersion equations to determine the index of refraction (
pharmacopeias demand that spectrophotometers perform according to strict regulatory requirements encompassing factors such as stray light[17] and wavelength accuracy.
A narrower spectral bandwidth provides higher resolution and accuracy, but also requires more time and energy to scan the entire spectrum.
A wider spectral bandwidth allows for faster and easier scanning, but may result in lower resolution and accuracy, especially for samples with overlapping absorption peaks.
It is important to have a monochromatic source of radiation for the light incident on the sample cell to enhance the linearity of the response.
The spectral bandwidth is measured as the number of wavelengths transmitted at half the maximum intensity of the light leaving the monochromator.
If this bandwidth is comparable to (or more than) the width of the absorption peak of the sample component, then the measured extinction coefficient will not be accurate.
If a significant amount of the light passed through the sample contains wavelengths that have much lower extinction coefficients than the nominal one, the instrument will report an incorrectly low absorbance.
In practice the concentration of the sample or the optical path length must be adjusted to place the unknown absorbance within a range that is valid for the instrument.
Sometimes an empirical calibration function is developed, using known concentrations of the sample, to allow measurements into the region where the instrument is becoming non-linear.
A more complex instrument with a double monochromator would have a stray light level corresponding to about 6 AU, which would therefore allow measuring a much wider absorbance range.
If cells of different path lengths are available, testing if this relationship holds true is one way to judge if absorption flattening is occurring.
If UV-Vis spectrophotometry is used in quantitative chemical analysis then the results are additionally affected by uncertainty sources arising from the nature of the compounds and/or solutions that are measured.
Mettler Toledo developed a single beam array spectrophotometer that allows fast and accurate measurements over the UV-Vis range.
The light source consists of a Xenon flash lamp for the ultraviolet (UV) as well as for the visible (VIS) and near-infrared wavelength regions covering a spectral range from 190 up to 1100 nm.
The lamp flashes are focused on a glass fiber which drives the beam of light onto a cuvette containing the sample solution.
The spectrograph consists of a diffraction grating that separates the light into the different wavelengths, and a CCD sensor to record the data, respectively.
The most widely applicable cuvettes are made of high quality fused silica or quartz glass because these are transparent throughout the UV, visible and near infrared regions.
As such, they are used in the forensic laboratory to analyze the dyes and pigments in individual textile fibers,[26] microscopic paint chips[27] and the color of glass fragments.
They are also used in materials science and biological research and for determining the energy content of coal and petroleum source rock by measuring the vitrinite reflectance.
In addition, ultraviolet–visible spectrophotometry can be used to determine the thickness, along with the refractive index and extinction coefficient of thin films.