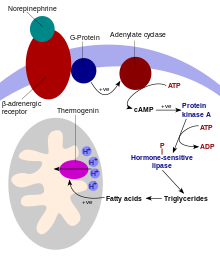
Thermogenin
UCP1-mediated heat generation in brown fat uncouples the respiratory chain, allowing for fast substrate oxidation with a low rate of ATP production.
The lipase converts triacylglycerols into free fatty acids, which activate UCP1, overriding the inhibition caused by purine nucleotides (GDP and ADP).
During the termination of thermogenesis, thermogenin is inactivated and residual fatty acids are disposed of through oxidation, allowing the cell to resume its normal energy-conserving state.
Substantiation for this modelling of UCP1 on ANT is found in the many conserved residues between the two proteins that are actively involved in the transportation of substrate across the membrane.
It wasn't until heat generation was adaptively selected for in placental mammal descendants of this common ancestor that UCP1 evolved its current function in brown adipose tissue to provide additional warmth.
[13] While UCP1 plays a key thermogenic role in wide range placental mammals, particularly those with small body size and those that hibernate, the UCP1 gene has lost functionality in several large-bodied lineages (e.g. horses, elephants, sea cows, whales and hyraxes) and lineages with low metabolic rates (e.g. pangolins, armadillos, sloths and anteaters).
[15] This further suggests that UCP1 had a different original purpose and in fact phylogenetic and sequence analyses indicate that UCP1 is likely a mutated form of a dicarboxylate carrier protein that adapted for thermogenesis in placental mammals.