Unfolded protein response
Once the sequence has “docked”, the protein continues translation, with the resultant strand being fed through the polypeptide translocator directly into the ER.
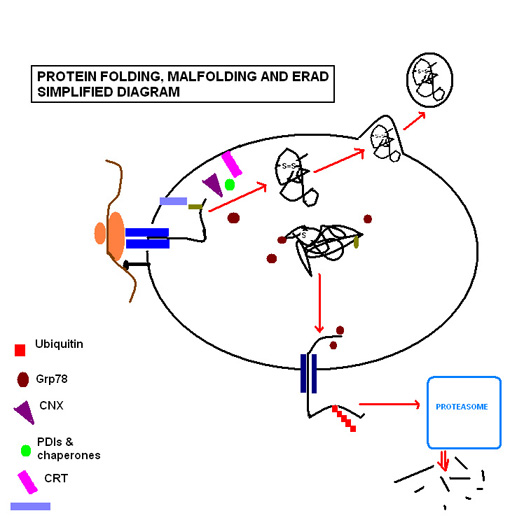
[10] Here it enters the ubiquitin-proteasome pathway, as it is tagged by multiple ubiquitin molecules, targeting it for degradation by cytosolic proteasomes.
[12] However, where circumstances cause a more global disruption to protein folding that overwhelms the ER's coping mechanisms, the UPR is activated.
It maintains specific transmembrane receptor proteins involved in initiation of the downstream signalling of the UPR in an inactive state by binding to their luminal domains.
It has been argued that the genetic and structural evidence supporting the model simply shows BiP dissociation to be merely correlated with Ire1 activation, rather than specifically causing it.
[14] An alternative model has been proposed, whereby unfolded proteins interact directly with the ER-lumenal domain of Ire1, causing oligomerization and transautophosphorylation.
[14] However these models are not mutually exclusive, it is also possible that both direct interaction of Ire1 with unfolded proteins and dissociation of BiP from IRE1 contribute to the activation of the Ire1 pathway.
This activates cellular stress signaling and inflammatory pathways because of the abnormal conditions disrupting ER homeostasis.
Muscular contraction during exercise causes calcium to be released from the sarcoplasmic reticulum (SR), a specialized ER network in skeletal muscles.
Therefore, this UPR pathway mediates changes in muscles that have undergone endurance training by making them more resistant to fatigue and protecting them from future stress.
[24] In conditions of prolonged stress, the goal of the UPR changes from being one that promotes cellular survival to one that commits the cell to a pathway of apoptosis.
However, the point at which the 'apoptotic switch' is activated has not yet been determined, but it is a logical consideration that this should be beyond a certain time period in which resolution of the stress has not been achieved.
[28] Endoplasmic reticulum stress was reported to play a major role in non‐alcoholic fatty liver disease (NAFLD) induction and progression.
High fat diet fed rats showed increased ER stress markers CHOP, XBP1, and GRP78.
For instance, the UPR is up-regulated in an inherited form of dilated cardiomyopathy due to a mutation in gene encoding the Phospholamban protein.
[30] Further activation proved therapeutic in a human induced pluripotent stem cell model of PLN mutant dilated cardiomyopathy.