VSEPR theory
[4][5] The idea of a correlation between molecular geometry and number of valence electron pairs (both shared and unshared pairs) was originally proposed in 1939 by Ryutaro Tsuchida in Japan,[6] and was independently presented in a Bakerian Lecture in 1940 by Nevil Sidgwick and Herbert Powell of the University of Oxford.
The electron pairs (or groups if multiple bonds are present) are assumed to lie on the surface of a sphere centered on the central atom and tend to occupy positions that minimize their mutual repulsions by maximizing the distance between them.
For example, when there are two electron pairs surrounding the central atom, their mutual repulsion is minimal when they lie at opposite poles of the sphere.
Through handling, balloons acquire a slight surface electrostatic charge that results in the adoption of roughly the same geometries when they are tied together at their stems as the corresponding number of electron pairs.
Based on the steric number and distribution of Xs and Es, VSEPR theory makes the predictions in the following tables.
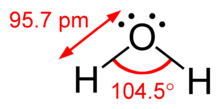
For example, the description of AX2E1 as a bent molecule means that the three atoms AX2 are not in one straight line, although the lone pair helps to determine the geometry.
The lone pairs on transition metal atoms are usually stereochemically inactive, meaning that their presence does not change the molecular geometry.
For example, the hexaaquo complexes M(H2O)6 are all octahedral for M = V3+, Mn3+, Co3+, Ni2+ and Zn2+, despite the fact that the electronic configurations of the central metal ion are d2, d4, d6, d8 and d10 respectively.
The Kepert model predicts the following geometries for coordination numbers of 2 through 9: The methane molecule (CH4) is tetrahedral because there are four pairs of electrons.
This is referred to as an AX3E type molecule because the lone pair is represented by an E.[1]: 410–417 By definition, the molecular shape or geometry describes the geometric arrangement of the atomic nuclei only, which is trigonal-pyramidal for NH3.
The shapes of heavier Group 14 element alkyne analogues (RM≡MR, where M = Si, Ge, Sn or Pb) have been computed to be bent.
[20][21][22] One example of the AX2E2 geometry is molecular lithium oxide, Li2O, a linear rather than bent structure, which is ascribed to its bonds being essentially ionic and the strong lithium-lithium repulsion that results.
[24] Burford et al showed through X-ray diffraction studies that Cl3Al–O–PCl3 has a linear Al–O–P bond angle and is therefore a non-VSEPR molecule.
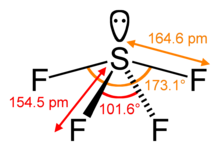
[25] Some AX6E1 molecules, e.g. xenon hexafluoride (XeF6) and the Te(IV) and Bi(III) anions, TeCl2−6, TeBr2−6, BiCl3−6, BiBr3−6 and BiI3−6, are octahedral, rather than pentagonal pyramids, and the lone pair does not affect the geometry to the degree predicted by VSEPR.
[13]: 214 The Kepert model predicts that ML4 transition metal molecules are tetrahedral in shape, and it cannot explain the formation of square planar complexes.
The explanation of the shape of square planar complexes involves electronic effects and requires the use of crystal field theory.
[13]: 562–4 Some transition metal complexes with low d electron count have unusual geometries, which can be ascribed to d subshell bonding interaction.
[29] Gillespie found that this interaction produces bonding pairs that also occupy the respective antipodal points (ligand opposed) of the sphere.
[30][4] This phenomenon is an electronic effect resulting from the bilobed shape of the underlying sdx hybrid orbitals.
[36] It has been proposed by Gillespie that this is also caused by bonding interaction of the ligands with the d subshell of the metal atom, thus influencing the molecular geometry.
[24][37] Relativistic effects on the electron orbitals of superheavy elements is predicted to influence the molecular geometry of some compounds.