Vaccine
[3][4] A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins.
Vaccines can be prophylactic (to prevent or alleviate the effects of a future infection by a natural or "wild" pathogen), or therapeutic (to fight a disease that has already occurred, such as cancer).
The World Health Organization (WHO) reports that licensed vaccines are currently available for twenty-five different preventable infections.
He used the phrase in 1798 for the long title of his Inquiry into the Variolae vaccinae Known as the Cow Pox, in which he described the protective effect of cowpox against smallpox.
[26] One type of primary immunodeficiency disorder resulting in genetic failure is X-linked agammaglobulinemia, in which the absence of an enzyme essential for B cell development prevents the host's immune system from generating antibodies to a pathogen.
[42] Other diseases such as rubella, polio, measles, mumps, chickenpox, and typhoid are nowhere near as common as they were a hundred years ago thanks to widespread vaccination programs.
[43] However, the difficulty of reaching all children, cultural misunderstandings, and disinformation have caused the anticipated eradication date to be missed several times.
[51] Host-("vaccinee")-related determinants that render a person susceptible to infection, such as genetics, health status (underlying disease, nutrition, pregnancy, sensitivities or allergies), immune competence, age, and economic impact or cultural environment can be primary or secondary factors affecting the severity of infection and response to a vaccine.
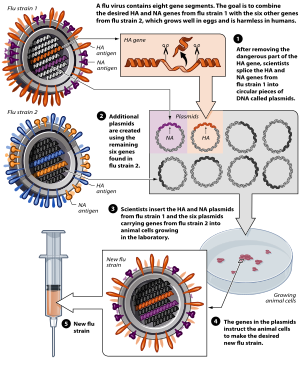
Many of these are active viruses that have been cultivated under conditions that disable their virulent properties, or that use closely related but less dangerous organisms to produce a broad immune response.
One example is the subunit vaccine against hepatitis B, which is composed of only the surface proteins of the virus (previously extracted from the blood serum of chronically infected patients but now produced by recombination of the viral genes into yeast).
[citation needed] Viral vector vaccines use a safe virus to insert pathogen genes in the body to produce specific antigens, such as surface proteins, to stimulate an immune response.
An extensive list of them provided in a sortable table and freely accessible is available at a US Centers for Disease Control and Prevention web page.
A vaccine licensure occurs after the successful conclusion of the development cycle and further the clinical trials and other programs involved through Phases I–III demonstrating safety, immunoactivity, immunogenetic safety at a given specific dose, proven effectiveness in preventing infection for target populations, and enduring preventive effect (time endurance or need for revaccination must be estimated).
[107] Vaccines developed for multinational distribution via the United Nations Children's Fund (UNICEF) require pre-qualification by the WHO to ensure international standards of quality, safety, immunogenicity, and efficacy for adoption by numerous countries.
Also during this stage, the proposed manufacturing facility is examined by expert reviewers for GMP compliance, and the label must have a compliant description to enable health care providers' definition of vaccine-specific use, including its possible risks, to communicate and deliver the vaccine to the public.
[111] In the United States, the Advisory Committee on Immunization Practices, which recommends schedule additions for the Centers for Disease Control and Prevention, recommends routine vaccination of children against[112] hepatitis A, hepatitis B, polio, mumps, measles, rubella, diphtheria, pertussis, tetanus, HiB, chickenpox, rotavirus, influenza, meningococcal disease and pneumonia.
[122] This increase, particularly in the number of different vaccines administered to children before entry into schools may be due to government mandates and support, rather than economic incentive.
[123] According to the World Health Organization, the biggest barrier to vaccine production in less developed countries has not been patents, but the substantial financial, infrastructure, and workforce requirements needed for market entry.
[127] There is a global scarcity of personnel with the right combination of skills, expertise, knowledge, competence and personality to staff vaccine production lines.
Cultured mammalian cells are expected to become increasingly important, compared to conventional options such as chicken eggs, due to greater productivity and low incidence of problems with contamination.
Combination vaccines are expected to reduce the quantities of antigens they contain, and thereby decrease undesirable interactions, by using pathogen-associated molecular patterns.
Vaccines intended for oral administration need not be liquid, and as solids, they commonly are more stable and less prone to damage or spoilage by freezing in transport and storage.
A microneedle approach, which is still in stages of development, uses "pointed projections fabricated into arrays that can create vaccine delivery pathways through the skin".
[148][149] They found that some existing vaccines against pseudorabies (also termed Aujeszky's disease) had deletions in their viral genome (among which was the gE gene).
[citation needed] Considerable efforts are ongoing to apply the DIVA principle to a wide range of infectious diseases, such as classical swine fever,[152] avian influenza,[153] Actinobacillus pleuropneumonia[154] and Salmonella infections in pigs.
[162][163] Jenner extended his studies and, in 1798, reported that his vaccine was safe in children and adults, and could be transferred from arm-to-arm, which reduced reliance on uncertain supplies from infected cows.
[167] Vaccinology flourished in the twentieth century, which saw the introduction of several successful vaccines, including those against diphtheria, measles, mumps, and rubella.
Separately, Inovio Pharmaceuticals and GeneOne Life Science began tests of a different DNA vaccine against Zika in Miami.
In 2021, Katalin Karikó and Drew Weissman received Columbia University's Horwitz Prize for their pioneering research in mRNA vaccine technology.
[177] Factors that affect the trends of vaccine development include progress in translatory medicine, demographics, regulatory science, political, cultural, and social responses.