White blood cell differential
[8] Marked shifts in the proportions of these cell types, as measured by the automated or manual differential, can indicate various health conditions.
[10][11] The manual differential can also identify changes in the appearance of white blood cells, such as reactive lymphocytes,[12] or features such as toxic granulation and vacuolation in neutrophils.
[6] The result are then compared against reference ranges, which are defined by individual laboratories and may vary due to different patient populations and testing methods.
[15] To prevent clotting, the sample is drawn into a tube containing the anticoagulant compound ethylenediaminetetraacetic acid (EDTA).,[16] meaning blood that has not been centrifuged.
[1][9] If the manual differential shows findings suggestive of certain serious conditions, such as leukemia, the blood smear is referred to a physician (generally a hematologist or pathologist) for confirmation.
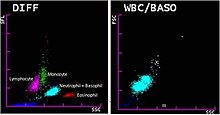
[34] Hematology analyzers measure various properties of white blood cells, such as impedance, light scattering parameters, and staining reactions.
This data is analyzed and plotted on a scattergram, forming distinct clusters which correspond to white blood cell types.
[26] If abnormal features or cell populations that the analyzer cannot identify are present, the instrument can flag the results for manual blood smear review.
Various cellular parameters, such as size, complexity and staining reactions, are measured and analyzed to identify cell populations.
[41] Hematology laboratories compensate for these issues by requiring a smear review when differential or CBC results fall outside certain numerical thresholds, regardless of the presence of analyzer flags.
[48] Outside of these conditions, increased neutrophil counts (neutrophilia) are associated with bacterial infection, inflammation, and various forms of physiological stress.
[49]Neutropenia, meaning a low neutrophil count, may occur as a response to drug treatment (especially chemotherapy)[51] or in certain infections, such as tuberculosis and Gram-negative sepsis.
[61] Low lymphocyte counts (lymphopenia) may be seen in infections such as HIV/AIDS, influenza and viral hepatitis, as well as in protein-energy malnutrition,[62] acute illnesses and drug reactions.
[59] In response to viral infections (especially infectious mononucleosis), lymphocytes may increase greatly in size, developing unusually shaped nuclei and large amounts of dark blue cytoplasm.
[64] Monocyte counts may be decreased (monocytopenia) in individuals who are receiving chemotherapy as well as those with aplastic anemia, severe burns, and AIDS.
[66][67] Eosinophil counts may be decreased in pregnancy and in response to physiological stress, inflammation or treatment with certain drugs, such as steroids and epinephrine.
[67] Basophilia and eosinophilia can occur along with other white blood cell abnormalities in chronic myeloid leukemia and other myeloproliferative disorders.
[69] A left shift, meaning an increase in band neutrophils or immature granulocytes, can indicate infection, inflammation or bone marrow disorders, although it can also be a normal finding in pregnancy.
[14] When present in significant quantities in the blood, immature granulocytes can indicate infection and inflammation,[11] as well as myeloproliferative disease, leukemia and other conditions affecting the marrow.
The presence of Auer rods inside blast cells indicates that they are of myeloid origin, which has important implications for leukemia treatment.
[10][76] Other morphologic features can provide information about the lineage of blast cells: for example, myeloblasts tend to be large with distinct nucleoli, while lymphoblasts can be smaller with a denser chromatin pattern.
Throughout the 18th and 19th centuries, improvements in microscope technology such as achromatic lenses allowed white blood cells and platelets to be counted in unstained samples.
[84] Dmitri Leonidovich Romanowsky improved on this technique in the 1890s by using a mixture of eosin and aged methylene blue, which produced a wide range of hues that was not present when either of the stains was used alone.
[85] By the early years of the 20th century, the white blood cell differential had become a common practice in the United States, but difficulties in interpreting the results cast doubt on the test's utility.
Schilling's monograph, Das Blutbild und seine klinische Verwertung (The Blood Picture and its Clinical Significance), was translated into English in 1926, and his neutrophil classification system quickly found acceptance in American laboratories.
Research into automating the differential count began in the 1970s and took two main approaches: digital image processing and flow cytometry.
Using technology developed in the 1950s and 60s to automate the reading of Pap smears, several models of image processing analyzers were produced.
[96] They were expensive, slow, and did little to reduce workload in the laboratory because they still required blood smears to be prepared and stained, so flow cytometry-based systems became more popular,[97][98] and by 1990, no digital image analyzers were commercially available in the United States or western Europe.
This analyzer was unpopular with hematology laboratories because it was labour-intensive to operate, but in the late 1980s to early 1990s similar systems were widely produced by other manufacturers such as Sysmex, Abbott, Roche and Beckman Coulter.