Withaferin A
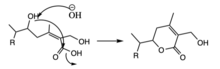
The 22nd and 26th carbons of the ergostane skeleton in withaferin A and related steroidal compounds are oxidized to form a six-membered delta lactone unit.
[3] A library of 2, 3-dihydro-3β-substituted derivatives are synthesized by regio/stereoselective Michael addition to ring A. NF-κB is a transcription factor that regulates many genes involved in cell survival, growth, immune response and angiogenesis.
Withaferin A inhibits NF-κB at a very low concentration by targeting the ubiquitin-mediated proteasome pathway (UPP) in endothelial cells.
[7] The biosynthesis of withaferin A uses enzymes such as squalene epoxidase (SQE), cycloartenol synthase (CAS), sterol methyl transferase (SMT), obtusifoliol-14 –demethylase (ODM).
[8] To produce withaferin A from 24-methylene cholesterol, the molecule undergoes several functional changes including formation of a ketone, epoxide, 2 hydroxyl groups, and lactone ring.