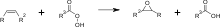Epoxy
Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance.
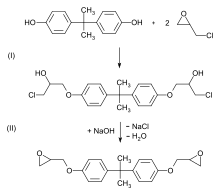
In 1946, Sylvan Greenlee, working for the Devoe & Raynolds Company (now part of Hexion Inc.[11]), patented resin derived from bisphenol-A and epichlorohydrin.
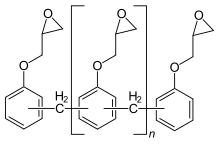
Epoxy resins are polymeric or semi-polymeric materials or an oligomer, and as such rarely exist as pure substances, since variable chain length results from the polymerisation reaction used to produce them.
Due to the low dielectric constants and the absence of chlorine, cycloaliphatic epoxides are often used to encapsulate electronic systems, such as microchips or LEDs.
If, however, they are used in larger proportions as reactive diluents, this often leads to reduced chemical and thermal resistance and to poorer mechanical properties of the cured epoxides.
However, reactivity is rather low compared to other classes of epoxy resin, and high temperature curing using suitable accelerators is normally required.
Some (non-crosslinked) epoxy resins with very high molar mass are added to engineering thermoplastics, again to achieve flame retardant properties.
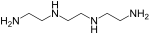
[26] However, good properties are obtained by reacting the linear epoxy resin with suitable curatives to form three-dimensional cross-linked thermoset structures.
[27] Curing of epoxy resins is an exothermic reaction and in some cases produces sufficient heat to cause thermal degradation if not controlled.
Temperature is sometimes increased in a step-wise fashion to control the rate of curing and prevent excessive heat build-up from the exothermic reaction.
The resulting network contains only ether bridges, and exhibits high thermal and chemical resistance, but is brittle and often requires elevated temperature for the curing process, so finds only niche applications industrially.
[citation needed] Epoxy resins may be thermally cured with anhydrides to create polymers with significant property retention at elevated temperatures for extended periods of time.
The resulting material has ether linkages and displays higher chemical and oxidation resistance than typically obtained by curing with amines or anhydrides.
[33] The applications include coatings, adhesives[34][35] and composite materials such as those using carbon fiber and fiberglass reinforcements (although polyester, vinyl ester, and other thermosetting resins are also used for glass-reinforced plastic).
[37] As with other classes of thermoset polymer materials, blending different grades of epoxy resin, as well as use of additives, plasticizers or fillers is common to achieve the desired processing or final properties, or to reduce cost.
[46] They found that the molecular reason for epoxy yellowing was a thermo-oxidative evolution of carbonyl groups in the polymeric carbon–carbon backbone via a nucleophilic radical attack.
Epoxy coatings are also widely used as primers to improve the adhesion of automotive and marine paints especially on metal surfaces where corrosion (rusting) resistance is important.
These high-performance adhesives are used in the construction of aircraft, automobiles, bicycles, boats, golf clubs, skis, snowboards, and other applications where high strength bonds are required.
This "plastic tooling" replaces metal, wood and other traditional materials, and generally improves the efficiency and either lowers the overall cost or shortens the lead-time for many industrial processes.
[49] In addition, for offshore and onshore wind energy installations, epoxy resins are used as protective coatings on steel towers, base struts and concrete foundations.
[50] Epoxy resin formulations are important in the electronics industry, and are employed in motors, generators, transformers, switchgear, bushings, insulators, printed wiring boards (PWB), and semiconductor encapsulants.
The largest volume type of circuit board—an "FR-4 board"—is a sandwich of layers of glass cloth bonded into a composite by an epoxy resin.
Transformer and inductor hot spots are greatly reduced, giving the component a stable and longer life than unpotted product.
Because of the better mechanical properties relative to the more common polyester resins, epoxies are used for commercial manufacture of components where a high strength/weight ratio is required.
[55] Water-soluble epoxies such as Durcupan[56][57] are commonly used for embedding electron microscope samples in plastic so they may be sectioned (sliced thin) with a microtome and then imaged.
Germany [50] is the largest market for epoxy resins in Europe, followed by Italy, France, the UK, Spain, the Netherlands and Austria.
Research is being done on innovative solutions such as using waste granite powders in epoxy resins and designing binders for coatings based on this.
[72] Liquid epoxy resins in their uncured state are mostly classed as irritant to the eyes and skin, as well as toxic to aquatic organisms.
Sensitization generally occurs due to repeated exposure (e.g. through poor working hygiene or lack of protective equipment) over a long period of time.
[74] Safe disposal also needs considering but usually involves deliberate curing to produce solid rather than liquid waste.