ZMapp
ZMapp was first used on humans during the Western African Ebola virus epidemic, having only been previously tested on animals and not yet subjected to a randomized controlled trial.
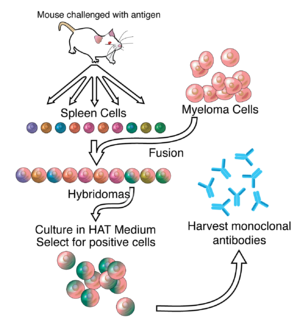
[5] The drug is composed of three monoclonal antibodies (mAbs), initially harvested from mice exposed to Ebola virus proteins, that have been chimerized with human constant regions.
[7] ZMapp is manufactured in the tobacco plant Nicotiana benthamiana in the bioproduction process known as "pharming" by Kentucky BioProcessing, a subsidiary of Reynolds American.
[14] MB-003 was created by scientists at the U.S. Army Medical Research Institute of Infectious Diseases, Gene Olinger, and Jamie Pettitt in collaboration with Mapp Biopharmaceutical with years of funding from US government agencies including the National Institute of Allergy and Infectious Disease, Biomedical Advanced Research and Development Authority, and the Defense Threat Reduction Agency.
[7] A study published in November 2013 found that EBOV-infected macaque monkeys survived after being given a therapy with a combination of three EBOV surface glycoprotein (EBOV-GP)-specific monoclonal antibodies (ZMAb) within 24 hours of infection.
[3] The development of these production methods was funded by the U.S. Defense Advanced Research Projects Agency as part of its bio-defense efforts following the 9/11 terrorist attacks.
[21][22] ZMapp was first used during the 2014 West Africa Ebola Virus outbreak, having not previously undergone any human clinical trials to determine its efficacy or potential risks.
[26] The lack of drugs and unavailability of experimental treatment in the most affected regions of the West African Ebola virus outbreak spurred some controversy.
[3] In August 2014, the World Health Organization called for convening a panel of medical authorities "to consider whether experimental drugs should be more widely released."
"[28] The National Institutes of Health announced on 27 February 2015 the commencement of a randomized controlled trial of ZMapp to be conducted in Liberia and the United States.
The panel agreed that "the benefits of ZMapp outweigh its risks" while noting that it presented logistical challenges, particularly that of requiring a cold chain for distribution and storage.