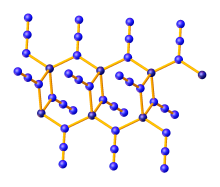
Zinc azide
It is a white, explosive solid that can be prepared by the protonolysis of diethylzinc with hydrazoic acid:[1] Zinc azide is a coordination polymer which crystallizes in three polymorphs, all of which feature tetrahedral zinc centers and bridging azide ligands.
α-Zn(N3)2 crystallizes in the monoclinic space group and is stable, while the other two polymorphs are metastable.
It is easily hydrolyzed, and attempts to prepare it in aqueous solution resulted in the precipitation of basic azides Zn(OH)2−x(N3)x (x = 0.9–1.0).
Both the α- and β-forms were found to be very friction- and shock-sensitive, violently exploding in blue flashes, but can be made to decompose slowly by gentle heating, giving off nitrogen gas.
In a sealed glass tube with inert atmosphere, this yields zinc nitride, Zn3N2.