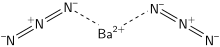Barium azide
Barium azide is an inorganic azide with the formula Ba(N3)2.
It is a barium salt of hydrazoic acid.
It is less sensitive to mechanical shock than lead azide.
Barium azide may be prepared by reacting sodium azide with a soluble barium salt:[6] Barium azide can be used to make azides of magnesium, sodium, potassium, lithium, rubidium and zinc with their respective sulfates.
[4] It can also be used as a source for high purity nitrogen by heating: This reaction liberates metallic barium, which is used as a getter in vacuum applications.