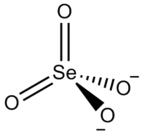
Zirconium selenate
Zirconium selenate is an inorganic compound with the chemical formula Zr(SeO4)2.
Its tetrahydrate can be obtained by the reaction of selenic acid and a saturated aqueous solution of zirconium oxychloride octahydrate (or zirconium hydroxide[2]).
The tetrahydrate belongs to the orthorhombic crystal system and is isostructural with Zr(SO4)2·4H2O.
It loses water when heated and becomes anhydrous at 220-230 °C.
[3] It reacts with potassium fluoride to obtain K2Zr(SeO4)2F2·3H2O.