Zirconium acetylacetonate
Zirconium acetylacetonate is the coordination complex with the formula Zr(C5H7O2)4.
It is a white solid that exhibits high solubility in nonpolar organic solvents, but not simple hydrocarbons.
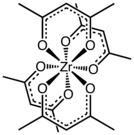
[1] The complex is prepared by treating zirconium oxychloride with acetylacetone:[1] The complex has a square antiprismatic geometry with eight nearly equivalent Zr-O bonds of length 2.19 Å.
[2] Compounds of high coordination number tend to be stereochemically nonrigid as indicated by the observation of one methyl signal by proton NMR spectroscopy.
[3] More volatile than Zr(acac)4 is the related complex of 1,1,1-trifluoroacetylacetonate.