Zopiclone
Zopiclone, sold under the brand name Imovane among others, is a nonbenzodiazepine, specifically a cyclopyrrolone, used to treat difficulty sleeping.
Although withdrawal symptoms from therapeutic doses of zopiclone and its isomers (i.e., eszopiclone) do not typically present with convulsions and are therefore not considered life-threatening, patients may experience such significant agitation or anxiety that they seek emergency medical attention.
[citation needed] In the United States, zopiclone is not commercially available,[3] although its active stereoisomer, eszopiclone, is.
Zopiclone is a controlled substance in the United States, Japan, Brazil, New Zealand and some European countries, and may be illegal to possess without a prescription.
[9][10][11][12] Zopiclone, similar to other benzodiazepines and nonbenzodiazepine hypnotic drugs, causes impairments in body balance and standing steadiness in individuals who wake up at night or the next morning.
Compared with the benzodiazepines, the nonbenzodiazepine sedative-hypnotics, such as zopiclone, offer few if any advantages in efficacy or tolerability in elderly persons.
Newer agents such as the melatonin receptor agonists may be more suitable and effective for the management of chronic insomnia in elderly people.
Long-term use of sedative-hypnotics for insomnia lacks an evidence base and is discouraged for reasons that include concerns about such potential adverse drug effects as cognitive impairment (anterograde amnesia), daytime sedation, motor incoordination, and increased risk of motor vehicle accidents and falls.
[19] The British National Formulary states adverse reactions as follows: "taste disturbance (some report a metallic taste); less commonly nausea, vomiting, dizziness, drowsiness, dry mouth, headache; rarely amnesia, confusion, depression, hallucinations, nightmares; very rarely light headedness, incoordination, paradoxical effects [...] and sleep-walking also reported".
Long-term users of hypnotic drugs for sleep disorders develop only partial tolerance to adverse effects on driving, with users of hypnotic drugs even after one year of use still showing an increased motor vehicle accident rate.
[23][24] Driving or operating machinery should be avoided after taking zopiclone as effects can carry over to the next day, including impaired hand-eye coordination.
[25][26] A double-blind study on the effect on performance of several hypnotic medications, relevant to military personnel who may have to be awakened to carry out duties, found that drugs listed in increasing order of performance impact duration were melatonin (with no impact), zaleplon, temazepam, and zopiclone.
[30] Zopiclone reduces the total amount of time spent in REM sleep as well as delaying its onset.
[37][38][39] Overdose of zopiclone may present with excessive sedation and depressed respiratory function that may progress to coma and possibly death.
[40] Zopiclone combined with alcohol, opiates, or other central nervous system depressants may be even more likely to lead to fatal overdoses.
Its mechanism of action is by binding to the benzodiazepine site and acting as a full agonist, which in turn positively modulates benzodiazepine-sensitive GABAA receptors and enhances GABA binding at the GABAA receptors to produce zopiclone's pharmacological properties.
It is rapidly and widely distributed to body tissues, including the brain, and is excreted in urine, saliva, and breast milk.
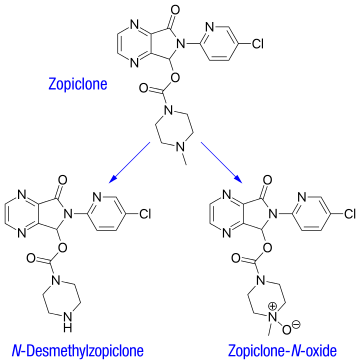
Zopiclone is partly extensively metabolized in the liver to form an active N-demethylated derivative (N-desmethylzopiclone) and an inactive zopiclone-N-oxide.
After oral administration of the racemic mixture, Cmax (time to maximum plasma concentration), area under the plasma time-concentration curve (AUC) and terminal elimination half-life values are higher for the dextrorotatory enantiomers, owing to the slower total clearance and smaller volume of distribution (corrected by the bioavailability), compared with the levorotatory enantiomer.
In urine, the concentrations of the dextrorotatory enantiomers of the N-demethyl and N-oxide metabolites are higher than those of the respective antipodes.
[69] In severe chronic kidney failure, the area under the curve value for zopiclone was larger and the half-life associated with the elimination rate constant longer, but these changes were not considered to be clinically significant.
[76][77] In 2005, the pharmaceutical company Sepracor of Marlborough, Massachusetts, began marketing the active stereoisomer eszopiclone under the name Lunesta in the United States.
Zopiclone is currently available off-patent in a number of European countries, Brazil, Canada, Hong Kong, and New Zealand.
The Compendium of Pharmaceuticals and Specialties recommends zopiclone prescriptions not exceed 7 to 10 days, owing to concerns of addiction, tolerance, and physical dependence.
Many drivers have blood levels far exceeding the therapeutic dose range and often in combination with alcohol, illegal, or addictive prescription drugs, suggesting a high degree of potential for non-medical use of benzodiazepines, zolpidem, and zopiclone.
It is frequently self-administered intravenously in studies on monkeys, suggesting a high risk of addictive potential.