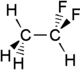
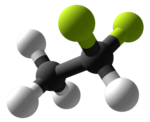
1,1-Difluoroethane
With a relatively low global warming potential (GWP) index of 124 and favorable thermophysical properties, 1,1-difluoroethane has been proposed as an environmentally friendly alternative to R134a.
[6] In addition, 1,1-difluoroethane is also commonly used in gas dusters and numerous other retail aerosol products, particularly those subject to stringent volatile organic compound (VOC) requirements.
Difluoroethane is an extremely flammable gas, which decomposes rapidly on heating or burning, producing toxic and irritating fumes, including hydrogen fluoride and carbon monoxide.
[14][15] Fatalities linked to difluoroethane abuse include actress Skye McCole Bartusiak, singer Aaron Carter and wrestler Mike Bell.
[16] Bitterants, added voluntarily to some brands to deter purposeful inhalation, are often not legally required; they do not negate or counteract difluoroethane's intoxicating effects.