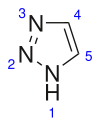
1,2,3-Triazole
Selectivity can be increased with metal catalysts, which have the added benefit of reacting without excessive or extensive heating.
This chemistry was expanded by Zhu et al. in 2018 wherein they report a two-step sequence from a terminal alkyne to 4-cyano 1,5-disubstituted triazoles.
[6] It is a surprisingly stable structure compared to other organic compounds with three adjacent nitrogen atoms.
However, flash vacuum pyrolysis at 500 °C leads to loss of molecular nitrogen (N2) leaving a three-member aziridine ring.
[7] Consequently it is an industrial building block for more complex chemicals, including pharmaceutical drugs such as mubritinib and tazobactam.