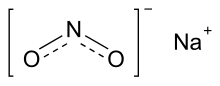
Sodium nitrite
It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.
[4] Sodium nitrite is an effective corrosion inhibitor and is used as an additive in industrial greases,[5] as an aqueous solution in closed loop cooling systems, and in a molten state as a heat transfer medium.
[6] Sodium nitrite is used to speed up the curing of meat,[7] inhibit the germination of Clostridium botulinum spores, and also impart an attractive pink color.
[8][9] Nitrite reacts with the meat myoglobin to cause color changes, first converting to nitrosomyoglobin (bright red), then, on heating, to nitrosohemochrome (a pink pigment).
[12] Through this research, sodium nitrite has been found to give taste and color to the meat and inhibit lipid oxidation that leads to rancidity, with varying degrees of effectiveness for controlling growth of disease-causing microorganisms.
[12] The ability of sodium nitrite to address the above-mentioned issues has led to production of meat with extended storage life and has improved desirable color and taste.
[19] In conjunction with salt and pH levels, sodium nitrite reduces the ability of Clostridium botulinum spores to grow to the point of producing toxin.
[7] Sodium nitrite has shown varying degrees of effectiveness for controlling growth of other spoilage or disease causing microorganisms.
[12] Although the inhibitory mechanisms are not well known, its effectiveness depends on several factors including residual nitrite level, pH, salt concentration, reductants present and iron content.
[19] It is generally agreed that sodium nitrite is not effective for controlling Gram-negative enteric pathogens such as Salmonella and Escherichia coli.
[19] Lipid peroxidation is considered to be a major reason for the deterioration of quality of meat products (rancidity and unappetizing flavors).
[19] Nitrite reacts with heme proteins and metal ions, neutralizing free radicals by nitric oxide (one of its byproducts).
[26] It is recommended only in severe cases of cyanide poisoning and has largely been replaced by use of hydroxocobalamin,[27] a form of vitamin B12, but given in much higher doses than needed nutritionally.
Side effects can include low blood pressure, headache, shortness of breath, loss of consciousness, and vomiting.
[42] In cases of suspected suicide involving sodium nitrite, it is critical that responding individuals administer immediate methylene blue.
[43][44][45] Methylene blue is the antidote to the methemoglobinemia caused by intentional ingestion of sodium nitrite as a suicide agent.
[49] The oftentimes severe methemoglobinemia found in sodium nitrite poisoning cases results in systemic hypoxia, metabolic acidosis, and cyanosis.
[64] Due to sodium nitrite's high level of toxicity to swine (Sus scrofa) it is now being developed in Australia to control feral pigs and wild boar.
[65][66] The sodium nitrite induces methemoglobinemia in swine, i.e. it reduces the amount of oxygen that is released from hemoglobin, so the animal will feel faint and pass out, and then die in a humane manner after first being rendered unconscious.
Dietary sources of nitrosamines include US cured meats preserved with sodium nitrite as well as the dried salted fish eaten in Japan.
[73] The nitrosonium ion formed in acidic nitrite solutions is commonly[74][75] mislabeled nitrous anhydride, an unstable nitrogen oxide that cannot exist in vitro.
[76] Ingesting nitrite under conditions that result in endogenous nitrosation has been classified as "probably carcinogenic to humans" by International Agency for Research on Cancer (IARC).
[79] One study has found a correlation between highly frequent ingestion of meats cured with pink salt and the COPD form of lung disease.
[4][84] A more commonly used method involves the general reaction of nitrogen oxides in alkaline aqueous solution, with the addition of a catalyst.