Propylene glycol
[7] Manufacturers use either non-catalytic high-temperature process at 200 °C (392 °F) to 220 °C (428 °F), or a catalytic method, which proceeds at 150 °C (302 °F) to 180 °C (356 °F) in the presence of ion exchange resin or a small amount of sulfuric acid or alkali.
Use of USP (US Pharmacopoeia) propylene glycol can reduce the risk of Abbreviated New Drug Application (ANDA) rejection.
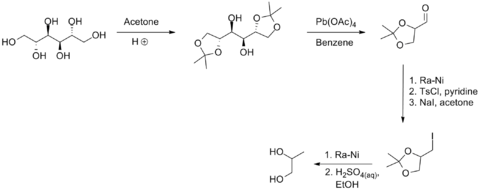
[7] A small-scale, nonbiological route from D-mannitol is illustrated in the following scheme:[12] Forty-five percent of propylene glycol produced is used as a chemical feedstock for the production of unsaturated polyester resins.
In this regard, propylene glycol reacts with a mixture of unsaturated maleic anhydride and isophthalic acid to give a copolymer.
[16] Propylene glycol is used in a variety of other edible items, such as baked goods, desserts, prepared meals, flavoring mixes, candy, popcorn, whipped dairy products, and soda.
Many pharmaceutical drugs which are insoluble in water utilize propylene glycol as a solvent and carrier; benzodiazepine tablets are one example.
A 50% water-diluted and heated solution is used for removal of icing accretions from the fuselages of commercial aircraft on the ground (de-icing), and 100% undiluted cold solution is used only on wings and tail surfaces of an aircraft in order to prevent ice accretion from forming during a specific period of time before takeoff (anti-icing).
[45][46] Toxicity generally occurs at plasma concentrations over 4 g/L, which requires extremely high intake over a relatively short period of time, or when used as a vehicle for drugs or vitamins given intravenously or orally in large bolus doses.
[47] It would be nearly impossible to reach toxic levels by consuming foods or supplements, which contain at most 1 g/kg of PG, except for alcoholic beverages in the US which are allowed 5 percent = 50 g/kg.
[8][13] Cases of propylene glycol poisoning are usually related to either inappropriate intravenous administration or accidental ingestion of large quantities by children.
[49] In a National Toxicology Program continuous breeding study, no effects on fertility were observed in male or female mice that received propylene glycol in drinking water at doses up to 10100 mg/kg bw/day.
A 2018 human volunteer study found that 10 male and female subjects undergoing 4 hours exposures to concentrations of up to 442 mg/m3 and 30 minutes exposures to concentrations of up to 871 mg/m3 in combination with moderate exercise did not show pulmonary function deficits, or signs of ocular irritation, with only slight symptoms of respiratory irritation reported.
[52] Propylene glycol has not caused sensitization or carcinogenicity in laboratory animal studies, nor has it demonstrated genotoxic potential.
[56] Recently, propylene glycol (commonly alongside glycerol) has been included as a carrier for nicotine and other additives in e-cigarette liquids, the use of which presents a novel form of exposure.
[57] According to a 2010 study, the concentrations of PGEs (counted as the sum of propylene glycol and glycol ethers) in indoor air, particularly bedroom air, has been linked to increased risk of developing numerous respiratory and immune disorders in children, including asthma, hay fever, eczema, and allergies, with increased risk ranging from 50% to 180%.
Adverse effects to intravenous administration of drugs that use propylene glycol as an excipient have been seen in a number of people, particularly with large bolus dosages.
[62] A high percentage (12–42%) of directly-injected propylene glycol is eliminated or secreted in urine unaltered depending on dosage, with the remainder appearing in its glucuronide-form.
The speed of renal filtration decreases as dosage increases,[63] which may be due to propylene glycol's mild anesthetic / CNS-depressant properties as an alcohol.
[67] However, it is prohibited for use in food for cats due to links to Heinz body formation and a reduced lifespan of red blood cells.
[70] Investigators believe that the incidence of allergic contact dermatitis to propylene glycol may be greater than 2% in patients with eczema or fungal infections, which are very common in countries with lesser sun exposure and lower-than-normal vitamin D balances.
[73] Because of its potential for allergic reactions and frequent use across a variety of topical and systemic products, propylene glycol was named the American Contact Dermatitis Society's Allergen of the Year for 2018.
Although propylene glycol has low toxicity, it exerts high levels of biochemical oxygen demand (BOD) during degradation in surface waters.
Large quantities of dissolved oxygen (DO) in the water column are consumed when microbial populations decompose propylene glycol.