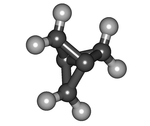
1.1.1-Propellane
The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.
[5] It starts with dibromocarbene addition to the alkene bond of 3-chloro-2-(chloromethyl)propene 6 followed by deprotonation by methyllithium and nucleophilic displacements in 7.
[1.1.1]Propellane spontaneously reacts with acetic acid to yield a methylidenecyclobutane ester (4 above).
[1.1.1]Propellane undergoes a polymerization reaction where the central C–C bond is split and connected to adjacent monomer units, resulting in staffanes.
[7] A radical polymerization initiated by methyl formate and benzoyl peroxide results in a distribution of oligomers.