2.2.2-Propellane
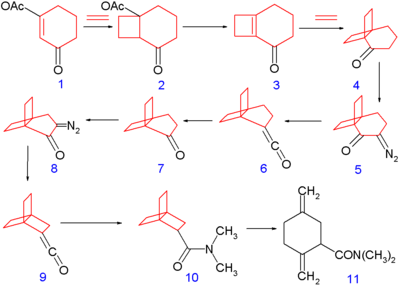
[2.2.2]Propellane was first synthesized in 1973 by the group of Philip Eaton (who had earlier obtained cubane),[2] according to the following scheme: The synthesis begins with photochemical [2+2]cycloaddition of ethene on the cyclohexene derivative 1 to produce the bicyclic compound 2, followed by elimination reaction with potassium t-butoxide of acetic acid to cyclobutene 3, followed by another cycloaddition with ethylene to 4.
This compound is converted to the diazo ketone 5 by deprotonation (using acetic acid and sodium methoxide) and reaction with tosyl azide.
Reaction with dimethylamine affords the [2.2.2]propellane backbone with a dimethylamide substituent 10.
The final product 10 was found to spontaneously isomerize in solution to the monocyclic amide 11, with a half-life of 28 minutes at room temperature.
A highly fluorinated [2.2.0]propellane was also synthesized by the group of David Lemal.