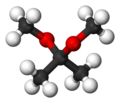
2,2-Dimethoxypropane
A colorless liquid, it is the product of the condensation of acetone and methanol.
Upon acid-catalyzed reaction, DMP reacts quantitatively with water to form acetone and methanol.
[2] This property can be used to accurately determine the amount of water in a sample, alternatively to the Karl Fischer method.
[3] DMP is specifically used to prepare acetonides:[4][5] Dimethoxypropane is an intermediate for the synthesis of 2-methoxypropene.
In histology, DMP is used for the dehydration of animal tissue.