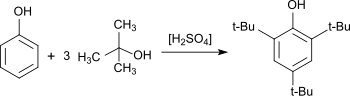
2,4,6-Tri-tert-butylphenol
In acidic media, the hydroxydienone is dealkylated with the cleavage of the tert-butyl group in the 4-position to the 2,6-di-tert-butylhydroquinone, which is oxidized to the end product 2,6-di-tert-butyl-1,4-benzoquinone.
[1][9][6] The 2,4,6-tri-tert-butylphenoxy radical forms blue crystals on cooling to -70 °C which are stable at room temperature for several weeks and only gradually turn yellow.
[10] The electron-rich 2,4,6-tri-tert-butylphenol can easily be oxidized to the phenoxy radical, which in the 4-position adds phenols,[11][12] as well as alcohols and thiols[13] to the corresponding cyclohexadienones.
The cyclohexadienones, also referred to in the literature as Chinolether, cleave the 4-position tert-butyl group upon heating under acidic conditions and aromatizes back to the substituted phenol.
2,4,6-TTBP is used as stabilizers, free-radical scavengers and antioxidants in technical applications, such as in fuels, hydraulic fluids and lubricating oils, as well as in elastomeric and thermoplastic polymers.
Because of its pronounced persistence, its high tendency for bioaccumulation and aquatic toxicity, 2,4,6-TTBP is only of low industrial use and is even forbidden, for example, in Japan.