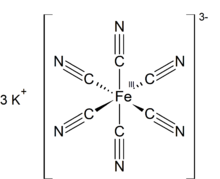Potassium ferricyanide
The compound is also used to harden iron and steel, in electroplating, dyeing wool, as a laboratory reagent, and as a mild oxidizing agent in organic chemistry.
Because potassium ferricyanide bleaches are environmentally unfriendly, short-lived, and capable of releasing hydrogen cyanide gas if mixed with high concentrations and volumes of acid, bleaches using ferric EDTA have been used in color processing since the 1972 introduction of the Kodak C-41 process.
In physiology experiments potassium ferricyanide provides a means increasing a solution's redox potential (E°' ~ 436 mV at pH 7).
Potassium ferricyanide is used to determine the ferric reducing power potential of a sample (extract, chemical compound, etc.).
To detect ferric (Fe3+) iron, potassium ferrocyanide is used instead in the Perls' Prussian blue staining method.
[14][15] Potassium ferricyanide has low toxicity, its main hazard being that it is a mild irritant to the eyes and skin.
However, under very strongly acidic conditions, highly toxic hydrogen cyanide gas is evolved, according to the equation: For example, it will react with diluted sulfuric acid under heating forming potassium sulfate, ferric sulfate and hydrogen cyanide.