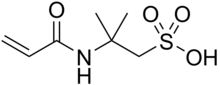
2-Acrylamido-2-methylpropane sulfonic acid
It is a reactive, hydrophilic, sulfonic acid acrylic monomer used to alter the chemical properties of wide variety of anionic polymers.
In the 1970s, the earliest patents using this monomer were filed for acrylic fiber manufacturing.
Today, there are over several thousands patents and publications involving use of AMPS in many areas including water treatment, oil field, construction chemicals, hydrogels for medical applications, personal care products, emulsion coatings, adhesives, and rheology modifiers.
AMPS is made by the Ritter reaction of acrylonitrile and isobutylene in the presence of sulfuric acid and water.
[2] The recent patent literature[3] describes batch and continuous processes that produce AMPS in high purity (to 99.7%) and improved yield (up to 89%, based on isobutene) with the addition of liquid isobutene to an acrylonitrile / sulfuric acid / phosphoric acid mixture at 40°C.