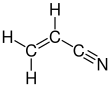
Acrylonitrile
[10] In the SOHIO process, propylene, ammonia, and air (oxidizer) are passed through a fluidized bed reactor containing the catalyst at 400–510 °C and 50–200 kPag.
The aqueous solution consists of acrylonitrile, acetonitrile, hydrocyanic acid, and ammonium sulfate (from excess ammonia).
A recovery column removes bulk water, and acrylonitrile and acetonitrile are separated by distillation.
[12][9] The glycerol route begins with its dehydration to acrolein, which undergoes ammoxidation to give acrylonitrile.
[5] The reaction of acrylonitrile with protic nucleophiles is a common route to a variety of specialty chemicals.
The process is called cyanoethylation: Typical protic nucleophiles are alcohols, thiols, and especially amines.
[21] Acrylonitrile increases cancer in high dose tests in male and female rats and mice[22] and induces apoptosis in human umbilical cord mesenchymal stem cells.
Routes of exposure include inhalation, oral, and to a certain extent dermal uptake (tested with volunteer humans and in rat studies).
The primary method is excretion in urine when acrylonitrile is metabolized by being directly conjugated to glutathione.
The other method is when acrylonitrile is enzymatically converted into 2-cyanoethylene oxide which will produce cyanide end products that ultimately form thiocyanate, which is excreted via urine.
[28] In March 1977 after a suit filed by the Natural Resources Defense Council the FDA rescinded approval of acrylonitrile bottles citing adverse effects on test animals.
[30][31] A large amount of acrylonitrile (approximately 6500 tons) leaked from an industrial polymer plant owned by Aksa Akrilik after the violent 17th August 1999 earthquake in Turkey.
Healthcare workers did not know about the health effects of acrylonitrile and tried to treat the victims with painkillers and IV fluids.
[33] In 2003, the owner of Aksa Akrilik died from lung cancer related to acrylonitrile exposure.
[36][37][38] Computer simulations suggest that on Titan conditions exist such that the compound could form structures similar to cell membranes and vesicles on Earth, called azotosomes.