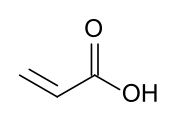
Acrylic acid
Acrylic acid was once manufactured by the hydrolysis of acrylonitrile, a material derived from propene by ammoxidation, but this route was abandoned because it cogenerates ammonium side products, which must be disposed of.
[7] Carboxylating ethylene to acrylic acid under supercritical carbon dioxide is thermodynamically possible, but efficient catalysts have not been developed.
Acrylic acid and its esters readily combine with themselves (to form polyacrylic acid) or other monomers (e.g. acrylamides, acrylonitrile, vinyl compounds, styrene, and butadiene) by reacting at their double bond, forming homopolymers or copolymers, which are used in the manufacture of various plastics, coatings, adhesives, elastomers, as well as floor polishes and paints.
The annual worldwide consumption of acrylic acid is projected to reach more than an estimated 8,000 kilotons by 2020.
More specifically, these are: Acrylic acid is severely irritating and corrosive to the skin and the respiratory tract.
[12] Ethyl acrylate was once used as synthetic food flavoring and was withdrawn by FDA possibly due to cancerogenic effects observed in lab animals.