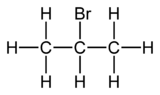
2-Bromopropane
It is used for introducing the isopropyl functional group in organic synthesis.
2-Bromopropane is prepared by heating isopropanol with hydrobromic acid.
It may be prepared in the ordinary manner of alkyl bromides, by reacting isopropanol with phosphorus and bromine,[4] or with phosphorus tribromide.
The bromine atom is at the secondary position, which allows the molecule to undergo dehydrohalogenation easily to give propene, which escapes as a gas and can rupture closed reaction vessels.
When this reagent is used in base catalyzed reactions, potassium carbonate should be used in place of sodium or potassium hydroxide.