Functional group
The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition.
Functional groups binding to a central atom in a coordination complex are called ligands.
In the common rule of thumb "like dissolves like", it is the shared or mutually well-interacting functional groups which give rise to solubility.
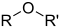
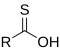
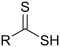
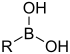
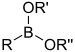
[3] In the formulas, the symbols R and R' usually denote an attached hydrogen, or a hydrocarbon side chain of any length, but may sometimes refer to any group of atoms.
Hydrocarbons are a class of molecule that is defined by functional groups called hydrocarbyls that contain only carbon and hydrogen, but vary in the number and order of double bonds.
There are also a large number of branched or ring alkanes that have specific names, e.g., tert-butyl, bornyl, cyclohexyl, etc.
Compounds that contain C-O bonds each possess differing reactivity based upon the location and hybridization of the C-O bond, owing to the electron-withdrawing effect of sp-hybridized oxygen (carbonyl groups) and the donating effects of sp2-hybridized oxygen (alcohol groups).
Compounds containing boron exhibit unique chemistry due to their having partially filled octets and therefore acting as Lewis acids.
methyllithium methylmagnesium chloride trimethylaluminium trimethylsilyl triflate note 1 Fluorine is too electronegative to be bonded to magnesium; it becomes an ionic salt instead.
There are some retained names, such as methylene for methanediyl, 1,x-phenylene for phenyl-1,x-diyl (where x is 2, 3, or 4),[5] carbyne for methylidyne, and trityl for triphenylmethyl.